Differential expression of claudin family proteins in mouse ovarian serous papillary epithelial adenoma in aging FSH receptor-deficient mutants
- PMID: 17217615
- PMCID: PMC1783714
- DOI: 10.1593/neo.06529
Differential expression of claudin family proteins in mouse ovarian serous papillary epithelial adenoma in aging FSH receptor-deficient mutants
Abstract
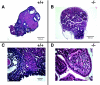
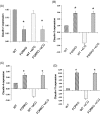
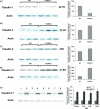
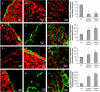
Ovarian cancer is a deadly disease with long latency. To understand the consequences of loss of follicle-stimulating hormone receptor (FSH-R) signaling and to explore why the atrophic and anovulatory ovaries of follitropin receptor knockout (FORKO) mice develop different types of ovarian tumors, including serous papillary epithelial adenoma later in life, we used mRNA expression profiling to gain a comprehensive view of misregulated genes. Using real-time quantitative reverse transcription-polymerase chain reaction, protein analysis, and cellular localization, we show, for the first time, in vivo evidence that, in the absence of FSH-R signaling, claudin-3, claudin-4, and claudin-11 are selectively upregulated, whereas claudin-1 decreases in ovarian surface epithelium and tumors in comparison to wild type. In vitro experiments using a mouse ovarian surface epithelial cell line derived from wild-type females reveal direct hormonal influence on claudin proteins. Although recent studies suggest that cell junction proteins are differentially expressed in ovarian tumors in women, the etiology of such changes remains unclear. Our results suggest an altered hormonal environment resulting from FSH-R loss as a cause of early changes in tight junction proteins that predispose the ovary to late-onset tumors that occur with aging. More importantly, this study identifies claudin-11 overexpression in mouse ovarian serous cystadenoma.
Figures
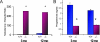

Similar articles
-
Aberrant expression of PDGF ligands and receptors in the tumor prone ovary of follitropin receptor knockout (FORKO) mouse.Carcinogenesis. 2006 May;27(5):903-15. doi: 10.1093/carcin/bgi305. Epub 2005 Dec 12. Carcinogenesis. 2006. PMID: 16344272
-
Ovarian pathology and high incidence of sex cord tumors in follitropin receptor knockout (FORKO) mice.Endocrinology. 2001 Aug;142(8):3673-84. doi: 10.1210/endo.142.8.8320. Endocrinology. 2001. PMID: 11459817
-
Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas.Clin Cancer Res. 2003 Jul;9(7):2567-75. Clin Cancer Res. 2003. PMID: 12855632
-
Chronology and complexities of ovarian tumorigenesis in FORKO mice: age-dependent gene alterations and progressive dysregulation of Major Histocompatibility Complex (MHC) Class I and II profiles.Mol Cell Endocrinol. 2010 Nov 25;329(1-2):37-46. doi: 10.1016/j.mce.2010.05.015. Epub 2010 Jul 6. Mol Cell Endocrinol. 2010. PMID: 20615452 Review.
-
FSH-FSHR3-stem cells in ovary surface epithelium: basis for adult ovarian biology, failure, aging, and cancer.Reproduction. 2015 Jan;149(1):R35-48. doi: 10.1530/REP-14-0220. Epub 2014 Sep 30. Reproduction. 2015. PMID: 25269615 Review.
Cited by
-
BRCA1-mediated signaling pathways in ovarian carcinogenesis.Funct Integr Genomics. 2012 Mar;12(1):63-79. doi: 10.1007/s10142-011-0251-2. Epub 2011 Sep 2. Funct Integr Genomics. 2012. PMID: 21887486
-
Ginger inhibits the invasion of ovarian cancer cells SKOV3 through CLDN7, CLDN11 and CD274 m6A methylation modifications.BMC Complement Med Ther. 2024 Apr 4;24(1):145. doi: 10.1186/s12906-024-04431-3. BMC Complement Med Ther. 2024. PMID: 38575994 Free PMC article.
-
β-catenin/Tcf-signaling appears to establish the murine ovarian surface epithelium (OSE) and remains active in selected postnatal OSE cells.BMC Dev Biol. 2012 Jun 8;12:17. doi: 10.1186/1471-213X-12-17. BMC Dev Biol. 2012. PMID: 22682531 Free PMC article.
-
Early alterations in ovarian surface epithelial cells and induction of ovarian epithelial tumors triggered by loss of FSH receptor.Neoplasia. 2007 Jun;9(6):521-31. doi: 10.1593/neo.07238. Neoplasia. 2007. PMID: 17603635 Free PMC article.
-
Transcriptomic Analysis of the Aged Nulliparous Mouse Ovary Suggests a Stress State That Promotes Pro-Inflammatory Lipid Signaling and Epithelial Cell Enrichment.Int J Mol Sci. 2023 Dec 30;25(1):513. doi: 10.3390/ijms25010513. Int J Mol Sci. 2023. PMID: 38203684 Free PMC article.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. Ca Cancer J Clin. 2005;55:10–30. - PubMed
-
- Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomark Prev. 2005;14:98–107. - PubMed
-
- Zanagnolo V, Sartori E, Trussardi E, Pasinetti B, Maggino T. Preservation of ovarian function, reproductive ability and emotional attitudes in patients with malignant ovarian tumors. Eur J Obstet Gynecol Reprod Biol. 2005;123:235–243. - PubMed
-
- Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18:S19–S32. - PubMed
-
- Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155:217–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
