Expression and effect of inhibition of the ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma
- PMID: 17217624
- PMCID: PMC1783715
- DOI: 10.1593/neo.05832
Expression and effect of inhibition of the ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma
Abstract
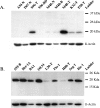
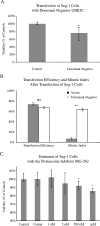
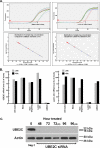
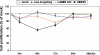
Ubiquitin-dependent proteolysis of cyclins plays a critical role in cell cycle progression and tumorigenesis. We examined the expression of ubiquitin-conjugating enzyme E2C (UBE2C) during progression from Barrett's metaplasia to esophageal adenocarcinoma (EA) and the effects of targeting this enzyme on EA-derived cell lines. Using oligonucleotide microarrays UBE2C expression was elevated in 73% (11 of 15) of EAs relative to Barrett's metaplasia. Tissue microarray showed elevated UBE2C in 70% (7 of 10) of dysplastic samples and in 87% (58 of 67) of tumors relative to metaplastic samples. Transfection of dominant-negative UBE2C into Seg-1 cells decreased proliferation (P = .04) and increased mitotic arrest compared to vector controls (63.5% vs 6.8%; P < .001). Transfection of UBE2C small interfering RNA also caused inhibiton of cell proliferation and distortion of the cell cycle, with maximal increase of G(2) cells (155% of mock cells) at 72 hours and of S-phase cells (308% of mock cells) at 24 hours. Treatment of Seg-1 cells with the proteasome inhibitor MG-262 (1 nM-1 microM) showed decreased proliferation (P = .02). EA-derived cells expressing UBE2C are sensitive to treatment with MG-262 and to silencing of UBE2C, suggesting that patients with EAs overexpressing UBE2C may benefit from agents targeting this ubiquitin-conjugating enzyme.
Figures


Similar articles
-
Bortezomib stabilizes mitotic cyclins and prevents cell cycle progression via inhibition of UBE2C in colorectal carcinoma.Am J Pathol. 2011 May;178(5):2109-20. doi: 10.1016/j.ajpath.2011.01.034. Am J Pathol. 2011. PMID: 21514426 Free PMC article.
-
Ubiquitin-conjugating enzyme E2C: a potential cancer biomarker.Int J Biochem Cell Biol. 2014 Feb;47:113-7. doi: 10.1016/j.biocel.2013.11.023. Epub 2013 Dec 17. Int J Biochem Cell Biol. 2014. PMID: 24361302 Review.
-
Silencing ubiquitin-conjugating enzyme 2C inhibits proliferation and epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma.FEBS J. 2019 Dec;286(24):4889-4909. doi: 10.1111/febs.15134. Epub 2019 Nov 29. FEBS J. 2019. PMID: 31715067
-
Identification of overexpressed genes in hepatocellular carcinoma, with special reference to ubiquitin-conjugating enzyme E2C gene expression.Int J Cancer. 2007 Jul 1;121(1):33-8. doi: 10.1002/ijc.22605. Int J Cancer. 2007. PMID: 17354233
-
Ubiquitin-conjugating enzyme UBE2C: molecular biology, role in tumorigenesis, and potential as a biomarker.Tumour Biol. 2012 Jun;33(3):723-30. doi: 10.1007/s13277-011-0291-1. Epub 2011 Dec 16. Tumour Biol. 2012. PMID: 22170434 Review.
Cited by
-
Osteopontin (OPN/SPP1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma.Oncotarget. 2015 Sep 8;6(26):22239-57. doi: 10.18632/oncotarget.4161. Oncotarget. 2015. PMID: 26068949 Free PMC article.
-
Identification of crucial genes associated with esophageal squamous cell carcinoma by gene expression profile analysis.Oncol Lett. 2018 Jun;15(6):8983-8990. doi: 10.3892/ol.2018.8464. Epub 2018 Apr 11. Oncol Lett. 2018. PMID: 29844815 Free PMC article.
-
Phospho-MED1-enhanced UBE2C locus looping drives castration-resistant prostate cancer growth.EMBO J. 2011 May 10;30(12):2405-19. doi: 10.1038/emboj.2011.154. EMBO J. 2011. PMID: 21556051 Free PMC article.
-
Gene expression profiling of connective tissue growth factor (CTGF) stimulated primary human tenon fibroblasts reveals an inflammatory and wound healing response in vitro.Mol Vis. 2011 Jan 8;17:53-62. Mol Vis. 2011. PMID: 21245951 Free PMC article.
-
High expression of UBE2C is associated with the aggressive progression and poor outcome of malignant glioma.Oncol Lett. 2016 Mar;11(3):2300-2304. doi: 10.3892/ol.2016.4171. Epub 2016 Feb 1. Oncol Lett. 2016. PMID: 26998166 Free PMC article.
References
-
- King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. - PubMed
-
- Townsley FM, Ruderman JV. Proteolytic ratchets that control progression through mitosis. Trends Cell Biol. 1998;8:238–244. - PubMed
-
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. - PubMed
-
- Lin Y, Hwang WC, Basavappa R. Structural and functional analysis of the human mitotic-specific ubiquitin-conjugating enzyme, UbcH10. J Biol Chem. 2002;277:21913–21921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
