Ubiquitination regulates PTEN nuclear import and tumor suppression
- PMID: 17218261
- PMCID: PMC1855245
- DOI: 10.1016/j.cell.2006.11.040
Ubiquitination regulates PTEN nuclear import and tumor suppression
Abstract
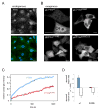
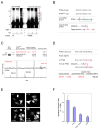
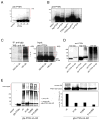
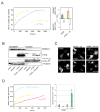
The PTEN tumor suppressor is frequently affected in cancer cells, and inherited PTEN mutation causes cancer-susceptibility conditions such as Cowden syndrome. PTEN acts as a plasma-membrane lipid-phosphatase antagonizing the phosphoinositide 3-kinase/AKT cell survival pathway. However, PTEN is also found in cell nuclei, but mechanism, function, and relevance of nuclear localization remain unclear. We show that nuclear PTEN is essential for tumor suppression and that PTEN nuclear import is mediated by its monoubiquitination. A lysine mutant of PTEN, K289E associated with Cowden syndrome, retains catalytic activity but fails to accumulate in nuclei of patient tissue due to an import defect. We identify this and another lysine residue as major monoubiquitination sites essential for PTEN import. While nuclear PTEN is stable, polyubiquitination leads to its degradation in the cytoplasm. Thus, we identify cancer-associated mutations of PTEN that target its posttranslational modification and demonstrate how a discrete molecular mechanism dictates tumor progression by differentiating between degradation and protection of PTEN.
Figures


Comment in
-
PTEN enters the nuclear age.Cell. 2007 Jan 12;128(1):25-8. doi: 10.1016/j.cell.2006.12.023. Cell. 2007. PMID: 17218252 Review.
References
-
- Bonneau D, Longy M. Mutations of the human PTEN gene. Hum Mutat. 2000;16:109–122. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Chi SG, Kim HJ, Park BJ, Min HJ, Park JH, Kim YW, Dong SH, Kim BH, Lee JI, Chang YW, et al. Mutational abrogation of the PTEN/MMAC1 gene in gastrointestinal polyps in patients with Cowden disease. Gastroenterology. 1998;115:1084–1089. - PubMed
-
- Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11:703–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

