Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny
- PMID: 17218346
- PMCID: PMC2802949
- DOI: 10.1093/aob/mcl260
Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny
Abstract
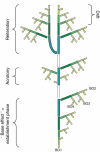
Background and aims: The architecture of a plant depends on the nature and relative arrangement of each of its parts; it is, at any given time, the expression of an equilibrium between endogenous growth processes and exogenous constraints exerted by the environment. The aim of architectural analysis is, by means of observation and sometimes experimentation, to identify and understand these endogenous processes and to separate them from the plasticity of their expression resulting from external influences.
Scope: Using the identification of several morphological criteria and considering the plant as a whole, from germination to death, architectural analysis is essentially a detailed, multilevel, comprehensive and dynamic approach to plant development. Despite their recent origin, architectural concepts and analysis methods provide a powerful tool for studying plant form and ontogeny. Completed by precise morphological observations and appropriated quantitative methods of analysis, recent researches in this field have greatly increased our understanding of plant structure and development and have led to the establishment of a real conceptual and methodological framework for plant form and structure analysis and representation. This paper is a summarized update of current knowledge on plant architecture and morphology; its implication and possible role in various aspects of modern plant biology is also discussed.
Figures




























Similar articles
-
Evolution and ecology of plant architecture: integrating insights from the fossil record, extant morphology, developmental genetics and phylogenies.Ann Bot. 2017 Nov 28;120(6):855-891. doi: 10.1093/aob/mcx113. Ann Bot. 2017. PMID: 29165551 Free PMC article. Review.
-
Curvature-driven spatial patterns in growing 3D domains: A mechanochemical model for phyllotaxis.PLoS One. 2018 Aug 16;13(8):e0201746. doi: 10.1371/journal.pone.0201746. eCollection 2018. PLoS One. 2018. PMID: 30114231 Free PMC article.
-
Molecular Mechanisms of Leaf Morphogenesis.Mol Plant. 2018 Sep 10;11(9):1117-1134. doi: 10.1016/j.molp.2018.06.006. Epub 2018 Jun 28. Mol Plant. 2018. PMID: 29960106 Review.
-
Persistent homology and the branching topologies of plants.Am J Bot. 2017 Mar;104(3):349-353. doi: 10.3732/ajb.1700046. Epub 2017 Mar 24. Am J Bot. 2017. PMID: 28341629 No abstract available.
-
Patterning at the shoot apical meristem and phyllotaxis.Curr Top Dev Biol. 2019;131:81-107. doi: 10.1016/bs.ctdb.2018.10.003. Epub 2018 Nov 27. Curr Top Dev Biol. 2019. PMID: 30612633 Review.
Cited by
-
Dwarfism mechanism in Malus clonal rootstocks.Planta. 2024 Nov 6;260(6):133. doi: 10.1007/s00425-024-04561-5. Planta. 2024. PMID: 39503906 Review.
-
Fine mapping of the gene controlling the weeping trait of Prunus persica and its uses for MAS in progenies.BMC Plant Biol. 2022 Sep 24;22(1):459. doi: 10.1186/s12870-022-03840-1. BMC Plant Biol. 2022. PMID: 36153492 Free PMC article.
-
Two decades of research with the GreenLab model in agronomy.Ann Bot. 2021 Feb 9;127(3):281-295. doi: 10.1093/aob/mcaa172. Ann Bot. 2021. PMID: 32969464 Free PMC article. Review.
-
Apple dwarfing rootstocks and interstocks affect the type of growth units produced during the annual growth cycle: precocious transition to flowering affects the composition and vigour of annual shoots.Ann Bot. 2008 Apr;101(5):679-87. doi: 10.1093/aob/mcn007. Epub 2008 Feb 9. Ann Bot. 2008. PMID: 18263898 Free PMC article.
-
Recruiting Conventional Tree Architecture Models into State-of-the-Art LiDAR Mapping for Investigating Tree Growth Habits in Structure.Front Plant Sci. 2018 Feb 20;9:220. doi: 10.3389/fpls.2018.00220. eCollection 2018. Front Plant Sci. 2018. PMID: 29515616 Free PMC article.
References
-
- Alpert P, Simms EL. The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evolutionary Ecology. 2002;16:285–297.
-
- Altman A, Goren R. Development of Citrus bud explants in culture. Journal of the American Society for Horticultural Science. 1978;103:120–123.
-
- Alvim P de T. Tree growth periodicity in tropical climates. In: Zimmermann MH, editor. Formation of wood in forest trees. New York: Academic Press; 1964. pp. 479–495.
-
- Ashby E. De la forme des feuilles et de leur rapport avec l'âge physiologique des plantes. Endeavour. 1949;T 8(29):18–25. - PubMed
-
- Atger C, Edelin C. Premières données sur l'architecture comparée des systèmes racinaires et caulinaires. Canadian Journal of Botany. 1994a;72:963–975.

