Molecular basis of the T- and L-type Ca2+ currents in canine Purkinje fibres
- PMID: 17218348
- PMCID: PMC2075395
- DOI: 10.1113/jphysiol.2006.127480
Molecular basis of the T- and L-type Ca2+ currents in canine Purkinje fibres
Abstract
This study examines the molecular basis for the T-type and L-type Ca(2+) currents in canine Purkinje cells. The I(CaT) in Purkinje cells was completely suppressed by 200 nM kurtoxin, a specific blocker of both Ca(v)3.1 and Ca(v)3.2 channels. Since only Ca(v)3.2 mRNA is expressed at high levels in Purkinje fibres, being approximately 100-fold more abundant than either Ca(v)3.1 or Ca(v)3.3 mRNAs, it is concluded that the Ca(v)3.2 gene encodes the bulk of the T-type Ca(2+) channels in canine Purkinje cells. This conclusion is consistent with the sensitivity of the current to blockade by Ni(2+) ions (K(D) = 32 microM). For L-type channels, Ca(v)1.2 mRNA was most abundant in Purkinje fibres but a significant level of Ca(v)1.3 mRNA expression was also found. A comparison of the sensitivity to blockade by isradipine of the L-type currents in Purkinje cells and ventricular epicardial myocytes, which only express Ca(v)1.2, suggests that the Ca(v)1.3 channels make, at most, a minor contribution to the L-type current in canine Purkinje cells.
Figures


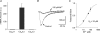
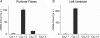
References
-
- Bohn G, Moosmang S, Conrad H, Ludwig A, Hofmann F, Klugbauer N. Expression of T- and L-type calcium channel mRNA in murine sinoatrial node. FEBS Lett. 2000;481:73–76. - PubMed
-
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. - PubMed
-
- Chuang RS, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat Neurosci. 1998;1:668–674. - PubMed
-
- Cole WC, Chartier D, Martin M, Leblanc N. Ca2+ permeation through Na+ channels in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol. 1997;273:H128–H137. - PubMed
-
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

