Investigation of domain formation in sphingomyelin/cholesterol/POPC mixtures by fluorescence resonance energy transfer and Monte Carlo simulations
- PMID: 17218467
- PMCID: PMC1864828
- DOI: 10.1529/biophysj.106.100107
Investigation of domain formation in sphingomyelin/cholesterol/POPC mixtures by fluorescence resonance energy transfer and Monte Carlo simulations
Abstract
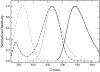
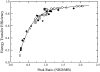
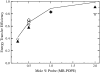
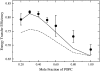
We have recently proposed a phase diagram for mixtures of porcine brain sphingomyelin (BSM), cholesterol (Chol), and 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) on the basis of kinetics of carboxyfluorescein efflux induced by the amphipathic peptide delta-lysin. Although that study indicated the existence of domains, phase separations in the micrometer scale have not been observed by fluorescence microscopy in BSM/Chol/POPC mixtures, though they have for some other sphingomyelins (SM). Here we examine the same BSM/Chol/POPC system by a combination of fluorescence resonance energy transfer (FRET) and Monte Carlo simulations. The results clearly demonstrate that domains are formed in this system. Comparison of the FRET experimental data with the computer simulations allows the estimate of lipid-lipid interaction Gibbs energies between SM/Chol, SM/POPC, and Chol/POPC. The latter two interactions are weakly repulsive, but the interaction between SM and Chol is favorable. Furthermore, those three unlike lipid interaction parameters between the three possible lipid pairs are sufficient for the existence of a closed loop in the ternary phase diagram, without the need to involve multibody interactions. The calculations also indicate that the largest POPC domains contain several thousand lipids, corresponding to linear sizes of the order of a few hundred nanometers.
Figures







References
-
- Veatch, S. L., and S. L. Keller. 2005. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94:148101. - PubMed
-
- Elliott, R., I. Szleifer, and M. Schick. 2006. Phase diagram of a ternary mixture of cholesterol and saturated and unsaturated lipids calculated from a microscopic model. Phys. Rev. Lett. 96:098101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

