Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis
- PMID: 17218989
- PMCID: PMC1764857
- DOI: 10.1172/JCI29539
Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis
Abstract
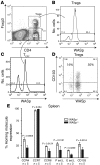
Wiskott-Aldrich syndrome protein (WASp) is essential for optimal T cell activation. Patients with WAS exhibit both immunodeficiency and a marked susceptibility to systemic autoimmunity. We investigated whether alterations in Treg function might explain these paradoxical observations. While WASp-deficient (WASp(-/-)) mice exhibited normal thymic Treg generation, the competitive fitness of peripheral Tregs was severely compromised. The total percentage of forkhead box P3-positive (Foxp3(+)) Tregs among CD4(+) T cells was reduced, and WASp(-/-) Tregs were rapidly outcompeted by WASp(+) Tregs in vivo. These findings correlated with reduced expression of markers associated with self-antigen-driven peripheral Treg activation and homing to inflamed tissue. Consistent with these findings, WASp(-/-) Tregs showed a reduced ability to control aberrant T cell activation and autoimmune pathology in Foxp3(-/-)Scurfy (sf) mice. Finally, WASp(+) Tregs exhibited a marked selective advantage in vivo in a WAS patient with a spontaneous revertant mutation, indicating that altered Treg fitness likely explains the autoimmune features in human WAS.
Figures
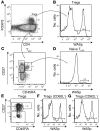


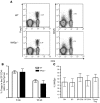

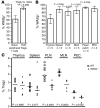
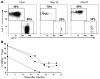
References
-
- Sullivan K.E., Mullen C.A., Blaese R.M., Winkelstein J.A. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J. Pediatr. 1994;125:876–885. - PubMed
-
- Thrasher A.J. WASp in immune-system organization and function. Nat. Rev. Immunol. . 2002;2:635–646. - PubMed
-
- Imai K., et al. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. - PubMed
-
- Dupuis-Girod S., et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–e627. - PubMed
-
- Burns S., Cory G.O., Vainchenker W., Thrasher A.J. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

