Efficacy of an osmotic pump delivered, GM-CSF-based tumor vaccine in the treatment of upper aerodigestive squamous cell carcinoma in rats
- PMID: 17219150
- PMCID: PMC11030275
- DOI: 10.1007/s00262-006-0271-2
Efficacy of an osmotic pump delivered, GM-CSF-based tumor vaccine in the treatment of upper aerodigestive squamous cell carcinoma in rats
Abstract
Purpose: Upper aerodigestive tract (UADT) cancer has not experienced significant overall survival improvement for over 20 years, and no successful treatments for systemic disease exist. Most patients with UADT cancer experience immune suppression, therefore immune restorative therapies may offer promise for these patients. We presently tested the efficacy of granulocyte macrophage-colony stimulating factor (GM-CSF) delivered via 28-day continuous infusion pump, in combination with irradiated tumor cells, in a flank model of UADT cancer.
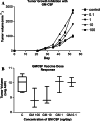
Methods: Five groups of rats were inoculated with syngeneic mucosally derived squamous carcinoma cells (FAT-7). Osmotic minipumps were implanted in the contralateral flank to deliver GM-CSF at 0 (PBS), 0.1, 1, 10, or 100 ng/day (n = 6 per group) for 28 days; 10(6) irradiated FAT-7 cells (ITC) were injected at the site of the GM-CSF infusion on days 0, 3, 7, 14, and 21 immune infiltrates in tumors were analyzed.
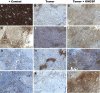
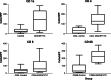
Results: Rats that received 10 or 100 ng/day GM-CSF/ITC had a significantly slower tumor growth rate compared to those who received 0, 0.1, or 1 ng/day (ANOVA, P < 0.01). There were increased CD 4+, CD 8+, and CD 68+ cells in tumors of GM-CSF/ITC treated animals over controls.
Conclusion: GM-CSF (10 or 100 ng/day) delivered locally via osmotic pump with ITC slows the growth rate of mucosally derived squamous cell carcinoma in rats while improving immune cell infiltrates. The efficacy of locally delivered GM-CSF immunotherapy in this model may be a first step toward this immunotherapy strategy for humans.
Figures

Similar articles
-
Effects of irradiated tumor vaccine and infusion of granulocyte-macrophage colony-stimulating factor and interleukin-12 on established gliomas in rats.Cancer Immunol Immunother. 2006 Jul;55(7):873-83. doi: 10.1007/s00262-005-0077-7. Epub 2005 Aug 27. Cancer Immunol Immunother. 2006. PMID: 16133106 Free PMC article.
-
Effects of continuous localized infusion of granulocyte-macrophage colony-stimulating factor and inoculations of irradiated glioma cells on tumor regression.J Neurosurg. 1999 Jun;90(6):1064-71. doi: 10.3171/jns.1999.90.6.1064. J Neurosurg. 1999. PMID: 10350253
-
Effects of combined granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2, and interleukin-12 based immunotherapy against intracranial glioma in the rat.J Neurooncol. 2004 Jan;66(1-2):39-49. doi: 10.1023/b:neon.0000013477.94568.0f. J Neurooncol. 2004. PMID: 15015768
-
Cellular immunotherapy using irradiated lung cancer cell vaccine co-expressing GM-CSF and IL-18 can induce significant antitumor effects.BMC Cancer. 2014 Jan 29;14:48. doi: 10.1186/1471-2407-14-48. BMC Cancer. 2014. Retraction in: BMC Cancer. 2023 Jul 3;23(1):612. doi: 10.1186/s12885-023-11123-7. PMID: 24475975 Free PMC article. Retracted.
-
Effects of irradiated tumor vaccine and continuous localized infusion of granulocyte-macrophage colony-stimulating factor on neuroblastomas in mice.J Pediatr Surg. 2002 Sep;37(9):1298-304. doi: 10.1053/jpsu.2002.34995. J Pediatr Surg. 2002. PMID: 12194120
Cited by
-
Enabling individualized therapy through nanotechnology.Pharmacol Res. 2010 Aug;62(2):57-89. doi: 10.1016/j.phrs.2009.12.011. Epub 2010 Jan 5. Pharmacol Res. 2010. PMID: 20045055 Free PMC article. Review.
-
Zero-order controlled release of ciprofloxacin-HCl from a reservoir-based, bioresorbable and elastomeric device.J Control Release. 2010 Sep 15;146(3):356-62. doi: 10.1016/j.jconrel.2010.05.036. Epub 2010 Jun 4. J Control Release. 2010. PMID: 20566343 Free PMC article.
References
-
- Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte/macrophage colony-stimulating factor stimulates potent, specific and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. - DOI - PMC - PubMed
-
- Levitsky HI, Montgomery J, Ahmadzadeh M, et al. Immunization with granulocyte/macrophage colony-stimulating factor, but not B7-1-transduced, lymphoma cells primes idiotype-specific T cells and generate potent systemic antitumor immunity. J Immunol. 1996;156:3858–3865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

