Recombinant polycistronic structure of hydantoinase process genes in Escherichia coli for the production of optically pure D-amino acids
- PMID: 17220246
- PMCID: PMC1828775
- DOI: 10.1128/AEM.02365-06
Recombinant polycistronic structure of hydantoinase process genes in Escherichia coli for the production of optically pure D-amino acids
Abstract
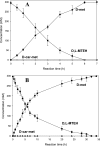
Two recombinant reaction systems for the production of optically pure D-amino acids from different D,L-5-monosubstituted hydantoins were constructed. Each system contained three enzymes, two of which were D-hydantoinase and D-carbamoylase from Agrobacterium tumefaciens BQL9. The third enzyme was hydantoin racemase 1 for the first system and hydantoin racemase 2 for the second system, both from A. tumefaciens C58. Each system was formed by using a recombinant Escherichia coli strain with one plasmid harboring three genes coexpressed with one promoter in a polycistronic structure. The D-carbamoylase gene was cloned closest to the promoter in order to obtain the highest level of synthesis of the enzyme, thus avoiding intermediate accumulation, which decreases the reaction rate. Both systems were able to produce 100% conversion and 100% optically pure D-methionine, D-leucine, D-norleucine, D-norvaline, D-aminobutyric acid, D-valine, D-phenylalanine, D-tyrosine, and D-tryptophan from the corresponding hydantoin racemic mixture. For the production of almost all D-amino acids studied in this work, system 1 hydrolyzed the 5-monosubstituted hydantoins faster than system 2.
Figures




Similar articles
-
Complete conversion of D,L-5-monosubstituted hydantoins with a low velocity of chemical racemization into D-amino acids using whole cells of recombinant Escherichia coli.Biotechnol Prog. 2002 Nov-Dec;18(6):1201-6. doi: 10.1021/bp0256162. Biotechnol Prog. 2002. PMID: 12467452
-
Overexpression and characterization of hydantoin racemase from Agrobacterium tumefaciens C58.Biochem Biophys Res Commun. 2003 Apr 4;303(2):541-7. doi: 10.1016/s0006-291x(03)00377-2. Biochem Biophys Res Commun. 2003. PMID: 12659852
-
Mechanism of stereospecific conversion of DL-5-substituted hydantoins to the corresponding L-amino acids by Pseudomonas sp. strain NS671.Biosci Biotechnol Biochem. 1997 Jan;61(1):185-7. doi: 10.1271/bbb.61.185. Biosci Biotechnol Biochem. 1997. PMID: 9028051
-
Optically pure alpha-amino acids production by the "Hydantoinase Process".Recent Pat Biotechnol. 2008;2(1):35-46. doi: 10.2174/187220808783330947. Recent Pat Biotechnol. 2008. PMID: 19075851 Review.
-
Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids.Curr Opin Biotechnol. 2001 Dec;12(6):559-63. doi: 10.1016/s0958-1669(01)00263-4. Curr Opin Biotechnol. 2001. PMID: 11849938 Review.
Cited by
-
The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection.Mol Plant Pathol. 2010 Sep;11(5):705-19. doi: 10.1111/j.1364-3703.2010.00625.x. Mol Plant Pathol. 2010. PMID: 20696007 Free PMC article. Review.
-
Potential application of N-carbamoyl-beta-alanine amidohydrolase from Agrobacterium tumefaciens C58 for beta-amino acid production.Appl Environ Microbiol. 2009 Jan;75(2):514-20. doi: 10.1128/AEM.01128-08. Epub 2008 Nov 14. Appl Environ Microbiol. 2009. PMID: 19011069 Free PMC article.
-
Engineering the third wave of biocatalysis.Nature. 2012 May 9;485(7397):185-94. doi: 10.1038/nature11117. Nature. 2012. PMID: 22575958 Review.
-
Application and microbial preparation of D-valine.World J Microbiol Biotechnol. 2016 Oct;32(10):171. doi: 10.1007/s11274-016-2119-z. Epub 2016 Aug 26. World J Microbiol Biotechnol. 2016. PMID: 27565781 Review.
-
Structural and biochemical characterisation of the N-carbamoyl-β-alanine amidohydrolase from Rhizobium radiobacter MDC 8606.FEBS J. 2023 Dec;290(23):5566-5580. doi: 10.1111/febs.16943. Epub 2023 Sep 8. FEBS J. 2023. PMID: 37634202 Free PMC article.
References
-
- Altenbuchner, J., M. Siemann-Herzberg, and C. Syldatk. 2001. Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids. Curr. Opin. Biotechnol. 12:559-563. - PubMed
-
- Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Short protocols in molecular biology. John Wiley and Sons, Inc., New York, NY.
-
- Chao, Y. P., H. Fu, T. E. Lo, P. T. Cheng, and J. J. Wang. 1999. One step production of d-p-hydroxyphenylglycine by recombinant Escherichia coli strains. Biotechnol. Prog. 15:1039-1045. - PubMed
-
- Clemente-Jimenez, J. M., S. Martinez-Rodriguez, L. Mingorance-Cazorla, S. De La Escalera-Hueso, F. J. Las Heras-Vazquez, and F. Rodriguez-Vico. 2003. Catalytic analysis of a recombinant d-hydantoinase from Agrobacterium tumefaciens. Biotechnol. Lett. 25:1067-1073. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

