Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi
- PMID: 17220265
- PMCID: PMC1828772
- DOI: 10.1128/AEM.02454-06
Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi
Abstract
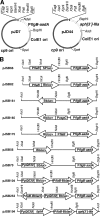
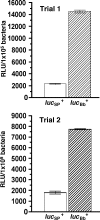
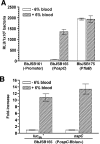
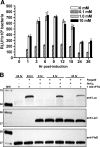
The development of new genetic systems for studying the complex regulatory events that occur within Borrelia burgdorferi is an important goal of contemporary Lyme disease research. Although recent advancements have been made in the genetic manipulation of B. burgdorferi, there still remains a paucity of basic molecular systems for assessing differential gene expression in this pathogen. Herein, we describe the adaptation of two powerful genetic tools for use in B. burgdorferi. The first is a Photinus pyralis firefly luciferase gene reporter that was codon optimized to enhance translation in B. burgdorferi. Using this modified reporter, we demonstrated an increase in luciferase expression when B. burgdorferi transformed with a shuttle vector encoding the outer surface protein C (OspC) promoter fused to the luciferase reporter was cultivated in the presence of fresh rabbit blood. The second is a lac operator/repressor system that was optimized to achieve the tightest degree of regulation. Using the aforementioned luciferase reporter, we assessed the kinetics and maximal level of isopropyl-beta-D-thiogalactopyranoside (IPTG)-dependent gene expression. This lac-inducible expression system also was used to express the gene carried on lp25 required for borrelial persistence in ticks (bptA). These advancements should be generally applicable for assessing further the regulation of other genes potentially involved in virulence expression by B. burgdorferi.
Figures

References
-
- Alam, J., and J. L. Cook. 1990. Reporter genes: application to the study of mammalian gene transcription. Anal. Biochem. 188:245-254. - PubMed
-
- Alverson, J., S. F. Bundle, C. D. Sohaskey, M. C. Lybecker, and D. S. Samuels. 2003. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol. Microbiol. 48:1665-1677. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

