Kgp and RgpB, but not RgpA, are important for Porphyromonas gingivalis virulence in the murine periodontitis model
- PMID: 17220315
- PMCID: PMC1828545
- DOI: 10.1128/IAI.01627-06
Kgp and RgpB, but not RgpA, are important for Porphyromonas gingivalis virulence in the murine periodontitis model
Abstract
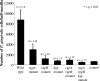
The contributions of three proteinase genes (rgpA, rgpB, and kgp) to the virulence of Porphyromonas gingivalis W50 were investigated in the murine periodontitis model. Mice were orally inoculated with eight doses (1 x 10(10) cells per dose) of rgpA, rgpB, kgp, rgpA rgpB, or rgpA rgpB kgp isogenic mutants, and the level of alveolar bone loss, immune response induced, and number of bacterial cells per half maxilla were compared with those of animals inoculated with wild-type P. gingivalis. The kgp, rgpB, rgpA rgpB, and rgpA rgpB kgp isogenic mutants induced significantly (P < 0.05) less bone loss than the rgpA isogenic mutant and the wild type did, and the virulence of the rgpA isogenic mutant and the wild type were not significantly different. Mice inoculated with the wild type or the rgpA isogenic mutant exhibited significantly (P < 0.01) more P. gingivalis cells per half maxilla than mice inoculated with rgpB, kgp, rgpA rgpB, and rgpA rgpB kgp isogenic mutants or nonchallenged mice did, as determined using real-time PCR. A significant positive correlation was found between the number of P. gingivalis cells detected per half maxilla and the amount of alveolar bone loss induced. Enzyme-linked immunosorbent assay results showed that each isogenic mutant and the wild type induced a predominant P. gingivalis antigen-specific immunoglobulin G3 (IgG3) response. Furthermore, the kgp and rgpA rgpB kgp isogenic mutants induced significantly (P < 0.05) lower IgG3 antibody responses than the responses induced by the wild type or the rgpA, rgpB, and rgpA rgpB isogenic mutants. The results suggest that the order in which the proteinases contribute to the virulence of P. gingivalis in the murine periodontitis model is Kgp > or = RgpB >> RgpA.
Figures
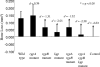

 ), anti-rgpA mutant sera (
), anti-rgpA mutant sera ( ), anti-rgpB mutant sera (□), anti-kgp mutant sera (
), anti-rgpB mutant sera (□), anti-kgp mutant sera ( ), anti-rgpA rgpB mutant sera (
), anti-rgpA rgpB mutant sera ( ), and anti-rgpA rgpB kgp mutant sera (
), and anti-rgpA rgpB kgp mutant sera ( ) and in sera from nonchallenged mice (
) and in sera from nonchallenged mice ( ) were analyzed by an ELISA. Antibody titers are expressed as the dilution factor which produced an absorbance fivefold greater than background in the ELISA. Each titer represents the mean plus standard deviation (error bar) of four values. Antibody titers that were significantly different (P < 0.05) from the antibody titer for the group inoculated with the P. gingivalis W50 wild-type strain are indicated by an asterisk.
) were analyzed by an ELISA. Antibody titers are expressed as the dilution factor which produced an absorbance fivefold greater than background in the ELISA. Each titer represents the mean plus standard deviation (error bar) of four values. Antibody titers that were significantly different (P < 0.05) from the antibody titer for the group inoculated with the P. gingivalis W50 wild-type strain are indicated by an asterisk.
Similar articles
-
Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model.Infect Immun. 2001 Dec;69(12):7527-34. doi: 10.1128/IAI.69.12.7527-7534.2001. Infect Immun. 2001. PMID: 11705929 Free PMC article.
-
The role of RgpA in the pathogenicity of Porphyromonas gingivalis in the murine periodontitis model.J Clin Periodontol. 2013 Oct;40(10):924-32. doi: 10.1111/jcpe.12139. Epub 2013 Aug 4. J Clin Periodontol. 2013. PMID: 23909600
-
The role of gingipains in the pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):111-8. doi: 10.1902/jop.2003.74.1.111. J Periodontol. 2003. PMID: 12593605 Review.
-
Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor.Infect Immun. 2002 Dec;70(12):6968-75. doi: 10.1128/IAI.70.12.6968-6975.2002. Infect Immun. 2002. PMID: 12438376 Free PMC article.
-
Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire.Curr Protein Pept Sci. 2003 Dec;4(6):409-26. doi: 10.2174/1389203033487009. Curr Protein Pept Sci. 2003. PMID: 14683427 Review.
Cited by
-
Cleavage of protease-activated receptors on an immortalized oral epithelial cell line by Porphyromonas gingivalis gingipains.Microbiology (Reading). 2009 Oct;155(Pt 10):3238-3246. doi: 10.1099/mic.0.029132-0. Epub 2009 Jul 16. Microbiology (Reading). 2009. PMID: 19608609 Free PMC article.
-
Outer Membrane Vesicle-Host Cell Interactions.Microbiol Spectr. 2019 Jan;7(1):10.1128/microbiolspec.psib-0001-2018. doi: 10.1128/microbiolspec.PSIB-0001-2018. Microbiol Spectr. 2019. PMID: 30681067 Free PMC article.
-
Involvement of an Skp-Like Protein, PGN_0300, in the Type IX Secretion System of Porphyromonas gingivalis.Infect Immun. 2015 Oct 26;84(1):230-40. doi: 10.1128/IAI.01308-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26502912 Free PMC article.
-
Inactive Gingipains from P. gingivalis Selectively Skews T Cells toward a Th17 Phenotype in an IL-6 Dependent Manner.Front Cell Infect Microbiol. 2017 Apr 27;7:140. doi: 10.3389/fcimb.2017.00140. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28497028 Free PMC article.
-
Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins.Periodontol 2000. 2010 Oct;54(1):15-44. doi: 10.1111/j.1600-0757.2010.00377.x. Periodontol 2000. 2010. PMID: 20712631 Free PMC article. Review. No abstract available.
References
-
- Aduse-Opoku, J., N. N. Davies, A. Gallagher, A. Hashim, H. E. Evans, M. Rangarajan, J. M. Slaney, and M. A. Curtis. 2000. Generation of Lys-gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg-gingipain protease activities. Microbiology 146:1933-1940. - PubMed
-
- Baker, P. J., R. T. Evans, and D. C. Roopenian. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 39:1035-1040. - PubMed
-
- Bosshardt, D. D., and N. P. Lang. 2005. The junctional epithelium: from health to disease. J. Dent. Res. 84:9-20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

