The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy
- PMID: 17220360
- PMCID: PMC1820924
- DOI: 10.1104/pp.106.093435
The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy
Abstract
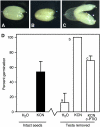
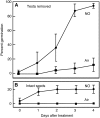
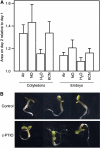
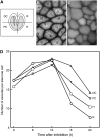
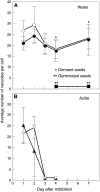
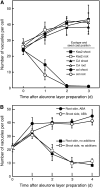
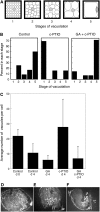
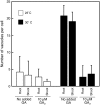
Seed dormancy is a common phase of the plant life cycle, and several parts of the seed can contribute to dormancy. Whole seeds, seeds lacking the testa, embryos, and isolated aleurone layers of Arabidopsis (Arabidopsis thaliana) were used in experiments designed to identify components of the Arabidopsis seed that contribute to seed dormancy and to learn more about how dormancy and germination are regulated in this species. The aleurone layer was found to be the primary determinant of seed dormancy. Embryos from dormant seeds, however, had a lesser growth potential than those from nondormant seeds. Arabidopsis aleurone cells were examined by light and electron microscopy, and cell ultrastructure was similar to that of cereal aleurone cells. Arabidopsis aleurone cells responded to nitric oxide (NO), gibberellin (GA), and abscisic acid, with NO being upstream of GA in a signaling pathway that leads to vacuolation of protein storage vacuoles and abscisic acid inhibiting vacuolation. Molecular changes that occurred in embryos and aleurone layers prior to germination were measured, and these data show that both the aleurone layer and the embryo expressed the NO-associated gene AtNOS1, but only the embryo expressed genes for the GA biosynthetic enzyme GA3 oxidase.
Figures



References
-
- Baskin J, Baskin C (1983) Seasonal changes in germination responses of buried seeds of Arabidopsis thaliana and ecological interpretations. Bot Gaz 144 540–543
-
- Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004. b) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219 847–855 - PubMed
-
- Bethke PC, Libourel IGL, Jones RL (2006. a) Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot 57 517–526 - PubMed
-
- Bethke PC, Libourel IGL, Reinohl V, Jones RL (2006. b) Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223 805–812 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

