De novo sphingolipid synthesis is essential for viability, but not for transport of glycosylphosphatidylinositol-anchored proteins, in African trypanosomes
- PMID: 17220466
- PMCID: PMC1828920
- DOI: 10.1128/EC.00283-06
De novo sphingolipid synthesis is essential for viability, but not for transport of glycosylphosphatidylinositol-anchored proteins, in African trypanosomes
Abstract
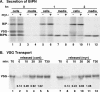
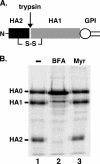
De novo sphingolipid synthesis is required for the exit of glycosylphosphatidylinositol (GPI)-anchored membrane proteins from the endoplasmic reticulum in yeast. Using a pharmacological approach, we test the generality of this phenomenon by analyzing the transport of GPI-anchored cargo in widely divergent eukaryotic systems represented by African trypanosomes and HeLa cells. Myriocin, which blocks the first step of sphingolipid synthesis (serine + palmitate --> 3-ketodihydrosphingosine), inhibited the growth of cultured bloodstream parasites, and growth was rescued with exogenous 3-ketodihydrosphingosine. Myriocin also blocked metabolic incorporation of [3H]serine into base-resistant sphingolipids. Biochemical analyses indicate that the radiolabeled lipids are not sphingomyelin or inositol phosphorylceramide, suggesting that bloodstream trypanosomes synthesize novel sphingolipids. Inhibition of de novo sphingolipid synthesis with myriocin had no adverse effect on either general secretory trafficking or GPI-dependent trafficking in trypanosomes, and similar results were obtained with HeLa cells. A mild effect on endocytosis was seen for bloodstream trypanosomes after prolonged incubation with myriocin. These results indicate that de novo synthesis of sphingolipids is not a general requirement for secretory trafficking in eukaryotic cells. However, in contrast to the closely related kinetoplastid Leishmania major, de novo sphingolipid synthesis is essential for the viability of bloodstream-stage African trypanosomes.
Figures
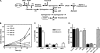
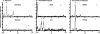



References
-
- Agusti, R., A. S. Couto, O. Campetella, A. C. C. Frasch, and R. M. de Lederkremer. 1998. Structure of the glycosylphosphatidylinositol-anchor of the trans-sialidase from Trypanosoma cruzi metacyclic trypomastigote forms. Mol. Biochem. Parasitol. 97:123-131. - PubMed
-
- Alexander, D. L., K. J. Schwartz, A. E. Balber, and J. D. Bangs. 2002. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 115:3253-3263. - PubMed
-
- Bangs, J. D., E. M. Brouch, D. M. Ransom, and J. L. Roggy. 1996. A soluble secretory reporter system in Trypanosoma brucei: studies on endoplasmic reticulum targeting. J. Biol. Chem. 271:18387-18393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

