Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2-/- mice
- PMID: 17220910
- PMCID: PMC2189719
- DOI: 10.1038/sj.bjp.0707116
Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2-/- mice
Abstract
Background and purpose: Mitochondrial aldehyde dehydrogenase (ALDH-2) has been shown to provide a pathway for bioactivation of organic nitrates and to be prone to desensitization in response to highly potent, but not to less potent, nitrates. We therefore sought to support the hypothesis that bioactivation by ALDH-2 critically depends on the number of nitrate groups within the nitrovasodilator.
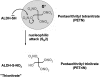
Experimental approach: Nitrates with one (PEMN), two (PEDN; GDN), three (PETriN; glyceryl trinitrate, GTN) and four (pentaerithrityl tetranitrate, PETN) nitrate groups were investigated. Vasodilatory potency was measured in isometric tension studies using isolated aortic segments of wild type (WT) and ALDH-2-/- mice. Activity of the cGMP-dependent kinase-I (reflected by levels of phosphorylated VAsodilator Stimulated Phosphoprotein, P-VASP) was quantified by Western blot analysis, mitochondrial dehydrogenase activity by HPLC. Following incubation of isolated mitochondria with PETN, PETriN-chromophore and PEDN, metabolites were quantified using chemiluminescence nitrogen detection and mass spectrometry.
Key results: Compared to WT, vasorelaxation in response to PETN, PETriN and GTN was attenuated about 10fold in ALDH-2-/- mice, identical to WT vessels preincubated with inhibitors of ALDH-2. Reduced vasodilator potency correlated with reduced P-VASP formation and diminished biotransformation of the tetranitrate- and trinitrate-compounds. None of these findings were observed for PEDN, GDN and PEMN.
Conclusions and implications: Our results support the crucial role of ALDH-2 in bioactivating highly reactive nitrates like GTN, PETN and PETriN. ALDH-2-mediated relaxation by organic nitrates therefore depends mainly on the number of nitrate groups. Less potent nitrates like PEDN, GDN and PEMN are apparently biotransformed by other pathways.
Figures
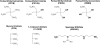



Similar articles
-
Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates.Mol Pharmacol. 2004 Dec;66(6):1372-82. doi: 10.1124/mol.104.002600. Epub 2004 Aug 26. Mol Pharmacol. 2004. PMID: 15331769
-
Mitochondrial oxidative stress and nitrate tolerance--comparison of nitroglycerin and pentaerithrityl tetranitrate in Mn-SOD+/- mice.BMC Cardiovasc Disord. 2006 Nov 8;6:44. doi: 10.1186/1471-2261-6-44. BMC Cardiovasc Disord. 2006. PMID: 17092343 Free PMC article.
-
A new class of organic nitrates: investigations on bioactivation, tolerance and cross-tolerance phenomena.Br J Pharmacol. 2009 Sep;158(2):510-20. doi: 10.1111/j.1476-5381.2009.00303.x. Epub 2009 Jun 25. Br J Pharmacol. 2009. PMID: 19563531 Free PMC article.
-
[Recent findings on nitrates: their action, bioactivation and development of tolerance].Dtsch Med Wochenschr. 2008 Oct;133(44):2277-82. doi: 10.1055/s-0028-1091272. Epub 2008 Oct 22. Dtsch Med Wochenschr. 2008. PMID: 18946854 Review. German.
-
Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress.Antioxid Redox Signal. 2015 Oct 10;23(11):899-942. doi: 10.1089/ars.2015.6376. Epub 2015 Sep 24. Antioxid Redox Signal. 2015. PMID: 26261901 Free PMC article. Review.
Cited by
-
Nitrates and nitrites in the treatment of ischemic cardiac disease.Cardiol Rev. 2010 Jul-Aug;18(4):190-7. doi: 10.1097/CRD.0b013e3181c8e14a. Cardiol Rev. 2010. PMID: 20539102 Free PMC article. Review.
-
The Endothelin Receptor Antagonist Macitentan Improves Isosorbide-5-Mononitrate (ISMN) and Isosorbide Dinitrate (ISDN) Induced Endothelial Dysfunction, Oxidative Stress, and Vascular Inflammation.Oxid Med Cell Longev. 2018 Dec 27;2018:7845629. doi: 10.1155/2018/7845629. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30687454 Free PMC article.
-
New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance.Clin Res Cardiol. 2008 Jan;97(1):12-20. doi: 10.1007/s00392-007-0588-7. Epub 2007 Oct 19. Clin Res Cardiol. 2008. PMID: 17938848 Review.
-
The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity.PLoS One. 2014 Nov 17;9(11):e112394. doi: 10.1371/journal.pone.0112394. eCollection 2014. PLoS One. 2014. PMID: 25402275 Free PMC article.
-
The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles.Br J Pharmacol. 2008 Sep;155(2):170-84. doi: 10.1038/bjp.2008.263. Epub 2008 Jun 23. Br J Pharmacol. 2008. PMID: 18574453 Free PMC article. Review.
References
-
- Abrams J. The role of nitrates in coronary heart disease. Arch Intern Med. 1995;155:357–364. - PubMed
-
- Bell FK, O'Neill JJ, Burgison RM. Determination of the oil/water distribution coefficients of glyceryl trinitrate and two similar nitrate esters. J Pharm Sci. 1963;52:637–639. - PubMed
-
- Daiber A, Oelze M, August M, Wendt M, Sydow K, Wieboldt H, et al. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res. 2004a;38:259–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

