Acid-promoted cyclization reactions of tetrahydroindolinones. Model studies for possible application in a synthesis of selaginoidine
- PMID: 17221972
- PMCID: PMC2475590
- DOI: 10.1021/jo0619783
Acid-promoted cyclization reactions of tetrahydroindolinones. Model studies for possible application in a synthesis of selaginoidine
Abstract
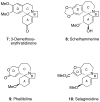
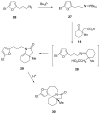
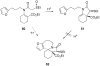
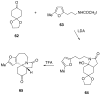
The synthesis of various substituted bicyclic lactams by an acid-induced Pictet-Spengler reaction of tetrahydroindolinones bearing tethered heteroaromatic rings is presented. The outcome of the cyclization depends on the position of the furan tether, tether length, nature of the tethered heteroaromatic ring, and the substituent group present on the 5-position of the tethered heteroaryl group. A one-pot procedure was developed to efficiently prepare tetrahydroindolinones containing tethered furan rings. In a typical example, the reaction of furanyl azide 26 with n-Bu3P delivered iminophosphorane 27, which was allowed to react with a 1-alkyl-(2-oxocyclohexyl)acetic acid to provide the desired furanyl-substituted tetrahydroindolinone system 29. Treatment of 29 with trifluoroacetic acid afforded the tetracyclic lactam skeleton 30 found in the alkaloid (+/-)-selaginoidine.
Figures
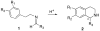



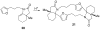






Similar articles
-
An aza-Wittig/pi-furan cyclization approach toward the homoerythrina alkaloid (+/-)-selaginoidine.Org Lett. 2005 Mar 31;7(7):1339-42. doi: 10.1021/ol0501323. Org Lett. 2005. PMID: 15787501
-
Enantioselective total synthesis of (+)-arborescidine C and related tetracyclic indole alkaloids using organocatalysis.Org Biomol Chem. 2017 Apr 18;15(16):3408-3412. doi: 10.1039/c7ob00473g. Org Biomol Chem. 2017. PMID: 28382347
-
Electrophilic-induced cyclization reaction of hexahydroindolinone derivatives and its application toward the synthesis of (+/-)-erysotramidine.J Org Chem. 2004 Nov 26;69(24):8209-18. doi: 10.1021/jo048647f. J Org Chem. 2004. PMID: 15549789
-
Synthesis of natural products with polycyclic systems.Chem Pharm Bull (Tokyo). 2013;61(3):251-7. doi: 10.1248/cpb.c12-01031. Chem Pharm Bull (Tokyo). 2013. PMID: 23449194 Review.
-
Advances and Strategies towards Synthesis of Aspidosperma Indole Alkaloids Goniomitine.Chem Biodivers. 2024 Jun;21(6):e202400416. doi: 10.1002/cbdv.202400416. Epub 2024 May 1. Chem Biodivers. 2024. PMID: 38587971 Review.
Cited by
-
Synthesis of tetrahydrofuro[3,2-c]pyridines via Pictet-Spengler reaction.Beilstein J Org Chem. 2023 Jun 30;19:991-997. doi: 10.3762/bjoc.19.74. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37404803 Free PMC article.
-
Brønsted Acid-Mediated Domino One-Pot Dual C-C Bond Formation: Chemoselective Synthesis of Fused Tricyclic Ketones.ACS Omega. 2018 Jan 9;3(1):218-228. doi: 10.1021/acsomega.7b01553. eCollection 2018 Jan 31. ACS Omega. 2018. PMID: 31457889 Free PMC article.
References
-
- Pictet A, Spengler T. Ber Dtsch Chem Ges. 1911;44:2030.
-
- Decker H, Becker P. Justus Liebigs Ann Chem. 1913;395:342.
-
-
For reviews, see: Czerwinski KM, Cook JM. Adv Heterocycl Nat Prod Synth. 1996;3:217.Cox ED, Cook JM. Chem Rev. 1995;95:1797.Waldmann H. Synlett. 1995:133.Rozwadowska MD. Heterocycles. 1994;39:903.Badia D, Dominguez E, Lete E, Villa MJ. Trends Heterocycl Chem. 1991;2:1.Ungemach F, Cook JM. Heterocycles. 1978;9:1089.Claret PA. In: Comprehensive Organic Chemistry. Barton D, Ollis WE, editors. Vol. 4. Pergomon; Oxford: 1979. p. 209.Gensler WJ. In: Heterocyclic Compounds. Elderfield RC, editor. Vol. 4. Wiley; New York: 1952. p. 353.Whaley WM, Govindachari TR. Org React. 1951;6:151.
-
-
- Brown RT. In: Indoles. Saxton JE, editor. Wiley-lnterscience; New York: 1983. Part 4.
- Bentley KW. Nat Prod Rep. 2004;21:395. and references cited therein. - PubMed
-
-
Strong Brönsted acids are most commonly employed to promote the Pictet-Spengler reaction, see: Dunetz JR, Ciccolini RP, Fröling M, Paap SM, Allen AJ, Holmes AB, Tester JW, Danheiser RL. Chem Commun. 2005:4465.Nakamura S, Tanaka M, Taniguchi T, Uchiyama M, Ohwada T. Org Lett. 2003;5:2087.Kuo F-M, Tseng M-C, Yen Y-H, Chu Y-H. Tetrahedron. 2004;60:12075.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

