Protein and DNA sequence determinants of thermophilic adaptation
- PMID: 17222055
- PMCID: PMC1769408
- DOI: 10.1371/journal.pcbi.0030005
Protein and DNA sequence determinants of thermophilic adaptation
Abstract
There have been considerable attempts in the past to relate phenotypic trait--habitat temperature of organisms--to their genotypes, most importantly compositions of their genomes and proteomes. However, despite accumulation of anecdotal evidence, an exact and conclusive relationship between the former and the latter has been elusive. We present an exhaustive study of the relationship between amino acid composition of proteomes, nucleotide composition of DNA, and optimal growth temperature (OGT) of prokaryotes. Based on 204 complete proteomes of archaea and bacteria spanning the temperature range from -10 degrees C to 110 degrees C, we performed an exhaustive enumeration of all possible sets of amino acids and found a set of amino acids whose total fraction in a proteome is correlated, to a remarkable extent, with the OGT. The universal set is Ile, Val, Tyr, Trp, Arg, Glu, Leu (IVYWREL), and the correlation coefficient is as high as 0.93. We also found that the G + C content in 204 complete genomes does not exhibit a significant correlation with OGT (R = -0.10). On the other hand, the fraction of A + G in coding DNA is correlated with temperature, to a considerable extent, due to codon patterns of IVYWREL amino acids. Further, we found strong and independent correlation between OGT and the frequency with which pairs of A and G nucleotides appear as nearest neighbors in genome sequences. This adaptation is achieved via codon bias. These findings present a direct link between principles of proteins structure and stability and evolutionary mechanisms of thermophylic adaptation. On the nucleotide level, the analysis provides an example of how nature utilizes codon bias for evolutionary adaptation to extreme conditions. Together these results provide a complete picture of how compositions of proteomes and genomes in prokaryotes adjust to the extreme conditions of the environment.
Conflict of interest statement
Figures


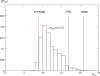
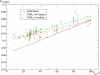



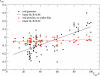
References
-
- Shakhnovich EI. Proteins with selected sequences fold into unique native conformation. Phys Rev Lett. 1994;72:3907–3910. - PubMed
-
- Jaenicke R, Bohm G. Stability of proteins in extreme environments. Curr Opin Struct Biol. 1998;8:738–748. - PubMed
-
- Jaenicke R. Stability and stabilization of globular proteins in solution. J Biotechnol. 2000;79:193–203. - PubMed
-
- Thompson MJ, Eisenberg D. Transproteomic evidence of a loop-deletion mechanism for enhancing protein thermostability. J Mol Biol. 1999;290:595–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

