Recovery of rat submandibular salivary gland function following removal of obstruction: a sialometrical and sialochemical study
- PMID: 17222209
- PMCID: PMC2517394
- DOI: 10.1111/j.1365-2613.2006.00500.x
Recovery of rat submandibular salivary gland function following removal of obstruction: a sialometrical and sialochemical study
Abstract
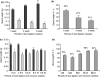
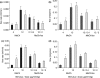
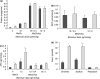
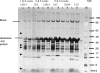
Functional recovery of the rat submandibular gland following ligation of the main excretory duct was examined. Rat submandibular glands were ligated for 1, 4 and 8 weeks using a micro-clip with a plastic tube. Micro-clips were removed and glands were allowed to recover for periods of 8, 16 and 24 weeks. Submandibular glands were stimulated with autonomimetic drugs (methacholine and isoprenaline) and salivas were collected from atrophic or de-ligated and contralateral control glands. Glands recovered almost full size (92% of control gland) following 24 weeks of de-ligation. Saliva volume secreted by ligated/de-ligated (RSM) and control (LSM) glands were similar with different doses of agonists. Protein output expressed per gram of tissue wet weight was similar from both ligated/de-ligated and control glands with all doses of agonist. Sodium and chloride levels were higher from de-ligated glands than contralateral control glands. Protein electrophoresis showed similar profiles of salivary proteins in all samples with some minor differences. Acinar cells in de-ligated glands showed a normal morphology, as indicated by light microscopy, whilst granular ductal cells were fewer and contained fewer secretory granules. Sodium potassium ATPase staining of striated ducts in de-ligated glands was similar to that of control glands. It can be concluded that rat submandibular glands can regenerate following severe atrophy and secrete normal amounts of saliva containing broadly a full profile of secretory proteins. In contrast to acinar cells, ductal cells appear not to recover full function.
Figures


Similar articles
-
Acute salivary gland hypofunction in the duct ligation model in the absence of inflammation.Oral Dis. 2008 Sep;14(6):520-8. doi: 10.1111/j.1601-0825.2007.01413.x. Epub 2008 Jan 22. Oral Dis. 2008. PMID: 18221457 Free PMC article.
-
Altered plasticity of the parasympathetic innervation in the recovering rat submandibular gland following extensive atrophy.Exp Physiol. 2009 Feb;94(2):213-9. doi: 10.1113/expphysiol.2008.045112. Epub 2008 Nov 21. Exp Physiol. 2009. PMID: 19028809 Free PMC article.
-
Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to decreased acinar volume and not impaired calcium signaling.Radiat Res. 1999 Feb;151(2):150-8. Radiat Res. 1999. PMID: 9952299
-
Salivary kallikrein excretion in hypertension.Klin Wochenschr. 1979 Oct 1;57(19):1047-52. doi: 10.1007/BF01479990. Klin Wochenschr. 1979. PMID: 392178 Review.
-
Duct ligation/de-ligation model: exploring mechanisms for salivary gland injury and regeneration.Front Cell Dev Biol. 2024 Jun 25;12:1399934. doi: 10.3389/fcell.2024.1399934. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38983787 Free PMC article. Review.
Cited by
-
Early markers of regeneration following ductal ligation in rat submandibular gland.Cell Tissue Res. 2008 May;332(2):227-35. doi: 10.1007/s00441-008-0588-6. Epub 2008 Mar 12. Cell Tissue Res. 2008. PMID: 18335244 Free PMC article.
-
Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation.Differentiation. 2010 Feb;79(2):120-30. doi: 10.1016/j.diff.2009.11.005. Epub 2010 Jan 6. Differentiation. 2010. PMID: 20056310 Free PMC article.
-
Ultrasound in Inflammatory and Obstructive Salivary Gland Diseases: Own Experiences and a Review of the Literature.J Clin Med. 2021 Aug 12;10(16):3547. doi: 10.3390/jcm10163547. J Clin Med. 2021. PMID: 34441850 Free PMC article. Review.
-
Is ductoplasty required following transoral sialolithotomy? - A case series.Eur Arch Otorhinolaryngol. 2025 Jul;282(7):3707-3713. doi: 10.1007/s00405-025-09324-w. Epub 2025 Mar 18. Eur Arch Otorhinolaryngol. 2025. PMID: 40102226
-
Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals.Stem Cell Reports. 2016 Jan 12;6(1):150-62. doi: 10.1016/j.stemcr.2015.11.009. Epub 2015 Dec 24. Stem Cell Reports. 2016. PMID: 26724906 Free PMC article.
References
-
- Abe K, Dawes C. The effect of electrical and pharmacological stimulation on the types of proteins secreted by the rat parotid and submandibular glands. Arch. Oral Biol. 1979;23:367–372. - PubMed
-
- Amerongen AVN, Veerman EC. Saliva – the defender of the oral cavity. Oral Dis. 2002;8:12–22. - PubMed
-
- Arneberg P. Quantitative determination of protein in saliva. Scand. J. Dent. Res. 1971;79:60–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

