Production of extracellular superoxide by human lymphoblast cell lines: comparison of electron spin resonance techniques and cytochrome C reduction assay
- PMID: 17222393
- PMCID: PMC1868485
- DOI: 10.1016/j.bcp.2006.12.012
Production of extracellular superoxide by human lymphoblast cell lines: comparison of electron spin resonance techniques and cytochrome C reduction assay
Abstract
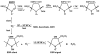
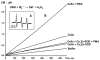
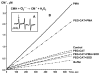
Superoxide production by NADPH oxidases plays an important role in the development and progression of cardiovascular disease (CVD). However, measurement of superoxide (O(2)(-)), a marker of oxidative stress, remains a challenging task in clinical and translational studies. In this study we analyzed O(2)(-) production in cultured human lymphoblast cell lines by three different methods: (a) superoxide dismutase (SOD)-inhibitable cytochrome C reduction, (b) spin trapping of superoxide with 5-(ethoxycarbonyl)-5-methyl-1-pyrroline N-oxide (EMPO) and 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO), and (c) using electron spin resonance (ESR) with the cell-permeable spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH). Lymphocytes were isolated and immortalized by an Epstein-Barr Virus (EBV)-transformation procedure. Superoxide was measured in cultured lymphoblast cell lines at baseline and upon stimulation with phorbol 12-myristate 13-acetate (PMA). Cytochrome C and the spin traps EMPO and DEPMPO detected two to five times less superoxide compared to CMH. Thus, CMH provided the most quantitative measurement of superoxide generation in human lymphoblast cell lines. Superoxide detection with CMH was linear dependent on cell concentration and was inhibited by SOD but not by catalase. Both cell-permeable polyethylene glycol (PEG)-SOD and extracellular Cu,Zn-SOD inhibited O(2)(-) detection by 90% in PMA-stimulated cells, suggesting a predominantly extracellular O(2)(-) generation in human lymphoblasts. Our study describes a new technique for O(2)(-) measurement in cultured human lymphoblasts using ESR and CMH. A highly sensitive in vitro measurement of O(2)(-) in human cell lines would allow investigators to study genotype/phenotype interactions in translational studies.
Figures


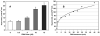



Similar articles
-
Superoxide dismutase versus ferricytochrome C: determining rate constants for the spin trapping of superoxide by cyclic nitrones.J Org Chem. 2004 Nov 26;69(24):8423-8. doi: 10.1021/jo0485029. J Org Chem. 2004. PMID: 15549816
-
Medium-throughput ESR detection of superoxide production in undetached adherent cells using cyclic nitrone spin traps.Free Radic Res. 2015;49(9):1122-8. doi: 10.3109/10715762.2015.1045504. Epub 2015 Jun 5. Free Radic Res. 2015. PMID: 25968949
-
Quantitative measurement of superoxide generation using the spin trap 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide.Anal Biochem. 1997 May 1;247(2):404-11. doi: 10.1006/abio.1997.2067. Anal Biochem. 1997. PMID: 9177705
-
ESR detection of intraphagosomal superoxide in polymorphonuclear leukocytes using 5-(diethoxyphosphoryl)-5-methyl-l-pyrroline-N-oxide.Free Radic Res. 2001 Jan;34(1):81-92. doi: 10.1080/10715760100300081. Free Radic Res. 2001. PMID: 11234998
-
Electron spin resonance spin-trapping detection of superoxide generated by neuronal nitric oxide synthase.Methods Enzymol. 1999;301:169-77. doi: 10.1016/s0076-6879(99)01080-0. Methods Enzymol. 1999. PMID: 9919565 Review.
Cited by
-
Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction.Biomaterials. 2013 Oct;34(31):7790-8. doi: 10.1016/j.biomaterials.2013.06.051. Epub 2013 Jul 13. Biomaterials. 2013. PMID: 23856052 Free PMC article.
-
Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase.Am J Physiol Regul Integr Comp Physiol. 2012 May 15;302(10):R1134-42. doi: 10.1152/ajpregu.00842.2010. Epub 2012 Mar 21. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22442197 Free PMC article.
-
Quantitation of spin probe-detectable oxidants in cells using electron paramagnetic resonance spectroscopy: To probe or to trap?Free Radic Biol Med. 2020 Jul;154:84-94. doi: 10.1016/j.freeradbiomed.2020.04.020. Epub 2020 May 4. Free Radic Biol Med. 2020. PMID: 32376456 Free PMC article.
-
EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines.Free Radic Res. 2011 Apr;45(4):417-30. doi: 10.3109/10715762.2010.540242. Epub 2010 Dec 3. Free Radic Res. 2011. PMID: 21128732 Free PMC article.
-
Hydrogen Sulfide Affects Radical Formation in the Hippocampus of LPS Treated Rats and the Effect of Antipsychotics on Hydrogen Sulfide Forming Enzymes in Human Cell Lines.Front Psychiatry. 2018 Oct 16;9:501. doi: 10.3389/fpsyt.2018.00501. eCollection 2018. Front Psychiatry. 2018. PMID: 30386265 Free PMC article.
References
-
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. - PubMed
-
- Zhang H, Joseph J, Vasquez-Vivar J, Karoui H, Nsanzumuhire C, Martasek P, et al. Detection of superoxide anion using an isotopically labeled nitrone spin trap: potential biological applications. FEBS Lett. 2000;473:58–62. - PubMed
-
- Frejaville C, Karoui H, Tuccio B, Le Moigne F, Culcasi M, Pietri S, et al. 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: a new efficient phosphorylated nitrone for the in vitro and in vivo spin trapping of oxygen-centered radicals. J Med Chem. 1995;38:258–65. - PubMed
-
- Tanigawa T, Kotake Y, Reinke LA. Spin trapping of superoxide from glass adherent polymorphonuclear leukocytes induced by N-formylmethionyl-leucyl-phenylalanine. Free Radic Res Commun. 1993;19:101–10. - PubMed
-
- Dikalov SI, Dikalova AE, Mason RP. Noninvasive diagnostic tool for inflammation-induced oxidative stress using electron spin resonance spectroscopy and an extracellular cyclic hydroxylamine. Arch Biochem Biophys. 2002;402:218–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

