In search of tumor suppressing functions of menin
- PMID: 17222504
- PMCID: PMC1919399
- DOI: 10.1016/j.mce.2006.12.032
In search of tumor suppressing functions of menin
Abstract
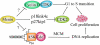
Human hereditary tumor syndromes serve as an ideal model for studying molecular pathways regulating tumorigenesis. Multiple endocrine neoplasia type 1 (MEN1), a human familial tumor syndrome, results from mutations in the Men1 gene. Men1 encodes a novel tumor suppressor, menin, of unknown biochemical function. Recently, significant progress has been made in identifying menin as a regulator of gene transcription, cell proliferation, apoptosis, and genome stability, leading to a new model of understanding menin's tumor-suppressing function. These findings suggest that menin's diverse functions depend on its association with chromatin and its control over gene transcription. This knowledge will likely be translated into new strategies to improve therapeutic interventions against MEN1 and other related cancers.
Figures

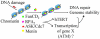
References
-
- Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, Burns AL. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–152. - PubMed
-
- Bale AE. Tumor models in Drosophila: application to MEN1. Journal of Internal Medicine. 2004;255:705.
-
- Bertolino P, Radovanovic I, Casse H, Aguzzi A, Wang ZQ, Zhang CX. Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech Dev. 2003;120:549–560. - PubMed
-
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

