The actin binding domain of ACF7 binds directly to the tetratricopeptide repeat domains of rapsyn
- PMID: 17222516
- PMCID: PMC1868462
- DOI: 10.1016/j.neuroscience.2006.11.047
The actin binding domain of ACF7 binds directly to the tetratricopeptide repeat domains of rapsyn
Abstract
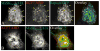
Formation of the neuromuscular junction requires the release of agrin from the presynaptic terminal of motor neurons. Clustering of acetylcholine receptors (AChRs) on the postsynaptic sarcolemma is initiated by agrin-dependent activation of the muscle-specific kinase. While the postsynaptic scaffolding protein rapsyn is vital for high density AChR aggregation, little is known about the mechanism through which AChRs are immobilized on the postsynaptic membrane. Ultrastructural and immunohistochemical studies of rat skeletal muscle have suggested that AChRs are anchored to a membrane-associated cytoskeleton that contains spectrin-like proteins and is thus similar to that of the human erythrocyte [Bloch RJ, Bezakova G, Ursitti JA, Zhou D, Pumplin DW (1997) A membrane skeleton that clusters nicotinic acetylcholine receptors in muscle. Soc Gen Physiol Ser 52:177-195]. We are studying a protein of the spectrin superfamily, ACF7 (also known as MACF), as a postsynaptic cytoskeletal component of the neuromuscular junction. ACF7 has multiple cytoskeleton-binding domains, including an N-terminal actin-binding domain that, we postulate, may interact with rapsyn, the scaffolding protein that binds directly to AChRs. To test this hypothesis, we co-expressed fragments of these molecules in cultured fibroblasts and assessed their co-distribution and interaction using confocal microscopy and co-immunoprecipitation. We demonstrate that the actin-binding domain of ACF7 specifically interacts with the tetratricopeptide repeat domains of rapsyn. Furthermore, we show using surface plasmon resonance and blot overlay that the actin-binding domain of ACF7 binds directly to rapsyn. These results suggest that, in mammalian skeletal muscle, AChRs are immobilized in the membrane through rapsyn-mediated anchoring to an ACF7-containing network that in turn is linked to the actin cytoskeleton.
Figures
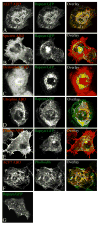
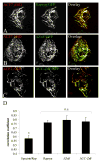

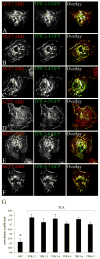

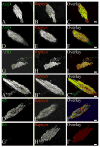
References
-
- Antolik C, Ursitti JA, Resneck WG, O’Neill AM, Lee PC, Pumplin DW, Bloch RJ. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006a. ACF7, a β-spectrin-like protein at clusters of acetylcholine receptors, associates with rapsyn. Program No. 30.2. 2006 Online.
-
- Antolik C, Catino DH, Resneck WG, Bloch RJ. The tetratricopeptide repeat domains of rapsyn bind directly to cytoplasmic sequences of the muscle-specific kinase. Neuroscience. 2006b;141:87–100. - PubMed
-
- Avnur Z, Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol. 1981;153:361–379. - PubMed
-
- Bartoli M, Ramarao MK, Cohen JB. Interactions of the rapsyn RING-H2 domain with dystroglycan. J Biol Chem. 2001;276:24911–24917. - PubMed
-
- Bezakova G, Bloch RJ. The zinc finger domain of the 43-kDa receptor-associated protein, rapsyn: role in acetylcholine receptor clustering. Mol Cell Neurosci. 1998;11:274–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

