The baculoviruses occlusion-derived virus: virion structure and function
- PMID: 17222693
- PMCID: PMC7112300
- DOI: 10.1016/S0065-3527(06)69003-9
The baculoviruses occlusion-derived virus: virion structure and function
Abstract
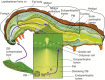
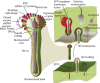
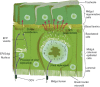
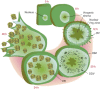
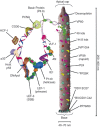
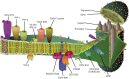
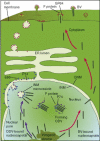
Baculoviruses play an important ecological role regulating the size of insect populations. For many years, baculoviruses have been applied as targeted biocontrol agents against forestry and agriculture pests. Baculovirus insecticides are effective against insect pests such as velvetbean caterpillar (Anticarsia gemmatalis), cotton bollworm (Helicoverpa zea), and gypsy moth (Lymantria dispar). Baculoviruses are transmitted to insects by the oral route mediated by the occlusion-derived virus (ODV). The ODV is also specialized to exploit the insect midgut that is one of the most extreme biological environments where the viruses are subject to caustic pH and digestive proteases. The molecular biology of the ODV reveals new frontiers in protein chemistry. Finally, ODVs establishes infection in insect gut tissues that are virtually nonsupportive to virus replication and which are continuously sloughed away. ODVs carry with them a battery of proteins that enable them to rapidly exploit and harness these unstable cells for virus replication.
Figures


References
-
- Abbas M., Boucias D.G. Interaction between nuclear polyhedrosis virus‐infected Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae and predator Podisus maculiventris (Say) (Hemiptera: Pentatomidae) Environ. Entomol. 1984;13(2):599–602.
-
- Aboussekhra A., Wood R.D. Detection of nucleotide excision repair incisions in human fibroblasts by immunostaining for PCNA. Exp. Cell Res. 1995;221(2):326–332. - PubMed
-
- Ackermann H.W., Smirnoff W.A. A morphological investigation of 23 baculoviruses. J. Invertebr. Pathol. 1983;41(3):269–280.
-
- Adams J.R., Bonami J.R. Atlas of invertebrate viruses. In: Adams J.R., Bonami J.R., editors. “Atlas of InvertebrateViruses”. CRC Press; Boca Raton: 1991.
-
- Adams J.R., McClintock J.T. Baculoviridae, nuclear polyhedrosis viruses Part 1. Nuclear polyhedrosis viruses of insects. In: Adams J.R., Bonami J.R., editors. “Atlas of Invertebrate Viruses”. CRC Press; Boca Raton: 1991. pp. 87–180. Chapter 6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

