AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients
- PMID: 17222792
- PMCID: PMC2748664
- DOI: 10.1016/j.ccr.2006.11.021
AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients
Abstract
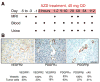
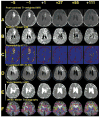
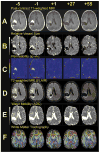
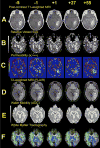
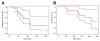
Using MRI techniques, we show here that normalization of tumor vessels in recurrent glioblastoma patients by daily administration of AZD2171-an oral tyrosine kinase inhibitor of VEGF receptors-has rapid onset, is prolonged but reversible, and has the significant clinical benefit of alleviating edema. Reversal of normalization began by 28 days, though some features persisted for as long as four months. Basic FGF, SDF1alpha, and viable circulating endothelial cells (CECs) increased when tumors escaped treatment, and circulating progenitor cells (CPCs) increased when tumors progressed after drug interruption. Our study provides insight into different mechanisms of action of this class of drugs in recurrent glioblastoma patients and suggests that the timing of combination therapy may be critical for optimizing activity against this tumor.
Figures
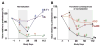

Comment in
-
Tracking normalization of brain tumor vasculature by magnetic imaging and proangiogenic biomarkers.Cancer Cell. 2007 Jan;11(1):6-8. doi: 10.1016/j.ccr.2006.12.008. Cancer Cell. 2007. PMID: 17222788 Free PMC article. Review.
-
Angiogenesis inhibition: the next frontier in multimodal therapy for glioblastoma multiforme.Nat Clin Pract Oncol. 2007 Aug;4(8):454-5. doi: 10.1038/ncponc0879. Epub 2007 Jul 3. Nat Clin Pract Oncol. 2007. PMID: 17609654 No abstract available.
References
-
- Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. - PubMed
-
- Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, DeWever J, Martinive P, Gregoire V, Feron O, Gallez B. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res. 2005;11:743–750. - PubMed
-
- Benner T, Wisco JJ, van der Kouwe AJ, Fischl B, Vangel MG, Hochberg FH, Sorensen AG. Comparison of manual and automatic section positioning of brain MR images. Radiology. 2006;239:246–254. - PubMed
-
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

