Negative regulation of ISG15 E3 ligase EFP through its autoISGylation
- PMID: 17222803
- PMCID: PMC1858649
- DOI: 10.1016/j.bbrc.2006.12.210
Negative regulation of ISG15 E3 ligase EFP through its autoISGylation
Abstract
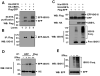
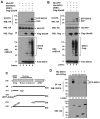
The function of ubiquitin-like protein ISG15 and protein modification by ISG15 (ISGylation) has been an enigma for many years. Recently, the research of ISGylation has been accelerated by the identification of the enzymes involved in the ISG15 conjugation process. Our previous study identified the interferon inducible protein EFP as an ISG15 isopeptide ligase (E3) for 14-3-3sigma. In this study, we show that ISG15 E3 ligase EFP can be modified by ISG15. Two ubiquitin E2 conjugating enzymes, UbcH6 and UbcH8, can support ISGylation of EFP. The Ring-finger domain of EFP is important for its ISGylation. Full-length EFP can enhance the ISGylation of Ring domain deleted EFP, indicating EFP can function as an ISG15 E3 ligase for itself. We also determined the ISGylation site of EFP and created its ISGylation resistant mutant EFP-K117R. Compared to the wild-type EFP, this mutant further increases the ISGylation of 14-3-3sigma. Thus we propose that autoISGylation of EFP negatively regulates its ISG15 E3 ligase activity for 14-3-3sigma.
Figures


References
-
- Haas AL, Ahrens P, Bright PM, Ankel H. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J.Biol.Chem. 1987;262:11315–11323. - PubMed
-
- Schwartz DC, Hochstrasser M. A superfamily of protein tags: ubiquitin. SUMO and related modifiers, Trends Biochem.Sci. 2003;28:321–328. - PubMed
-
- Farrell PJ, Broeze RJ, Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979;279:523–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

