Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids
- PMID: 17223150
- PMCID: PMC2710585
- DOI: 10.1016/j.virol.2006.11.031
Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids
Abstract
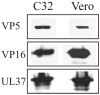
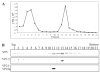
The assembly of the tegument of herpes simplex virus type 1 (HSV-1) is a complex process that involves a number of events at various sites within virus-infected cells. Our studies focused on determining whether tegument proteins, VP1/2 and UL37, are added to capsids located within the nucleus. Capsids were isolated from the nuclear fraction of HSV-1-infected cells and purified by rate-zonal centrifugation to separate B capsids (containing the scaffold proteins and no viral DNA) and C capsids (containing DNA and no scaffold proteins). Western blot analyses of these capsids indicated that VP1/2 associated primarily with C capsids and UL37 associated with B and C capsids. The results demonstrate that at least two of the tegument proteins of HSV-1 are associated with capsids isolated from the nuclear fraction, and these capsid-tegument protein interactions may represent initial events of the tegumentation process.
Figures





Similar articles
-
A null mutation in the gene encoding the herpes simplex virus type 1 UL37 polypeptide abrogates virus maturation.J Virol. 2001 Nov;75(21):10259-71. doi: 10.1128/JVI.75.21.10259-10271.2001. J Virol. 2001. PMID: 11581394 Free PMC article.
-
Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment.J Virol. 2014 Jun;88(11):5927-35. doi: 10.1128/JVI.00278-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24600000 Free PMC article.
-
Conserved Tryptophan Motifs in the Large Tegument Protein pUL36 Are Required for Efficient Secondary Envelopment of Herpes Simplex Virus Capsids.J Virol. 2016 May 12;90(11):5368-5383. doi: 10.1128/JVI.03167-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009950 Free PMC article.
-
Structure of the pseudorabies virus capsid: comparison with herpes simplex virus type 1 and differential binding of essential minor proteins.J Mol Biol. 2013 Sep 23;425(18):3415-28. doi: 10.1016/j.jmb.2013.06.034. Epub 2013 Jul 1. J Mol Biol. 2013. PMID: 23827137 Free PMC article.
-
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses.Viruses. 2015 Sep 18;7(9):5084-114. doi: 10.3390/v7092861. Viruses. 2015. PMID: 26393641 Free PMC article. Review.
Cited by
-
Assembly and Egress of an Alphaherpesvirus Clockwork.Adv Anat Embryol Cell Biol. 2017;223:171-193. doi: 10.1007/978-3-319-53168-7_8. Adv Anat Embryol Cell Biol. 2017. PMID: 28528444 Free PMC article. Review.
-
Herpes Simplex Virus 1 Small Capsomere-Interacting Protein VP26 Regulates Nucleocapsid Maturation.J Virol. 2017 Aug 24;91(18):e01068-17. doi: 10.1128/JVI.01068-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28679756 Free PMC article.
-
Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1.J Virol. 2009 Jan;83(1):105-16. doi: 10.1128/JVI.01032-08. Epub 2008 Oct 29. J Virol. 2009. PMID: 18971278 Free PMC article.
-
The incredible bulk: Human cytomegalovirus tegument architectures uncovered by AI-empowered cryo-EM.Sci Adv. 2024 Feb 23;10(8):eadj1640. doi: 10.1126/sciadv.adj1640. Epub 2024 Feb 23. Sci Adv. 2024. PMID: 38394211 Free PMC article.
-
Functional analysis of nuclear localization signals in VP1-2 homologues from all herpesvirus subfamilies.J Virol. 2014 May;88(10):5391-405. doi: 10.1128/JVI.03797-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574406 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

