Identification of the molecular switch that regulates access of 5alpha-DHT to the androgen receptor
- PMID: 17223255
- PMCID: PMC1857325
- DOI: 10.1016/j.mce.2006.12.007
Identification of the molecular switch that regulates access of 5alpha-DHT to the androgen receptor
Abstract
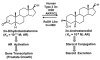
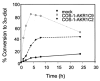
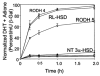
Pairs of hydroxysteroid dehydrogenases (HSDs) govern ligand access to steroid receptors in target tissues and act as molecular switches. By acting as reductases or oxidases, HSDs convert potent ligands into their cognate inactive metabolites or vice versa. This pre-receptor regulation of steroid hormone action may have profound effects on hormonal response. We have identified the HSDs responsible for regulating ligand access to the androgen receptor (AR) in human prostate. Type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) acts solely as a reductase to convert 5alpha-dihydrotestosterone (DHT), a potent ligand for the AR (K(d)=10(-11)M for the AR), to the inactive androgen 3alpha-androstanediol (K(d)=10(-6)M for the AR); while RoDH like 3alpha-HSD (a short-chain dehydrogenase/reductase (SDR)) acts solely as an oxidase to convert 3alpha-androstanediol back to 5alpha-DHT. Our studies suggest that aldo-keto reductase (AKRs) and SDRs function as reductases and oxidases, respectively, to control ligand access to nuclear receptors.
Figures


References
-
- Agarwal AK, Auchus RJ. Minireview: Cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology. 2005;146:2531–2538. - PubMed
-
- Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α,17β-diol to 5α-dihydrotestosterone: A potential therapeutic target for androgen dependent disease. Mol Endocrinol. 2006a;20:444–458. - PubMed
-
- Buaman DP, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006b Epub Ahead of Print September 7, 2006. - PubMed
-
- Biswas MG, Russell DW. Expression cloning and characterization of oxidative 17β– and 3α-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem. 1997;272:15959–15966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

