Atomic resolution structures of rieske iron-sulfur protein: role of hydrogen bonds in tuning the redox potential of iron-sulfur clusters
- PMID: 17223530
- PMCID: PMC1868424
- DOI: 10.1016/j.str.2006.11.012
Atomic resolution structures of rieske iron-sulfur protein: role of hydrogen bonds in tuning the redox potential of iron-sulfur clusters
Abstract
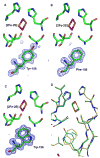
The Rieske [2Fe-2S] iron-sulfur protein of cytochrome bc(1) functions as the initial electron acceptor in the rate-limiting step of the catalytic reaction. Prior studies have established roles for a number of conserved residues that hydrogen bond to ligands of the [2Fe-2S] cluster. We have constructed site-specific variants at two of these residues, measured their thermodynamic and functional properties, and determined atomic resolution X-ray crystal structures for the native protein at 1.2 A resolution and for five variants (Ser-154-->Ala, Ser-154-->Thr, Ser-154-->Cys, Tyr-156-->Phe, and Tyr-156-->Trp) to resolutions between 1.5 A and 1.1 A. These structures and complementary biophysical data provide a molecular framework for understanding the role hydrogen bonds to the cluster play in tuning thermodynamic properties, and hence the rate of this bioenergetic reaction. These studies provide a detailed structure-function dissection of the role of hydrogen bonds in tuning the redox potentials of [2Fe-2S] clusters.
Conflict of interest statement
Figures
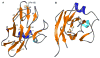

References
-
- Backes G, Mino Y, Loehr TM, Meyer TE, Cusanovich MA, Sweeney WV, Adman ET, Sanders-Loefr J. Environment of Fe4S4 clusters in ferredoxins and high-potential iron proteins. New information from x-ray crystallography and resonance Raman spectroscopy. J Am Chem Soc. 1991;113:2055–2064.
-
- Bard AJ, Faulkner LR. Electrochemical Methods. 2. New York: Wiley; 2001.
-
- Berry EA, Guergova-Kuras M, Huang LS, Crofts AR. Structure and function of cytochrome bc complexes. Annu Rev Biochem. 2000;69:1005–1075. - PubMed
-
- Bonisch H, Schmidt CL, Schafer G, Ladenstein R. The structure of the soluble domain of an archaeal Rieske iron-sulfur protein at 1.1 A resolution. J Mol Biol. 2002;319:791–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

