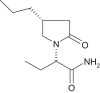
The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males
- PMID: 17223857
- PMCID: PMC2000585
- DOI: 10.1111/j.1365-2125.2006.02829.x
The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males
Abstract
Aims: The objective of the study was to evaluate the pharmacokinetics (and how they are affected by food), CNS pharmacodynamics and the adverse event profile of brivaracetam after single increasing doses.
Methods: Healthy males (n = 27, divided into three alternating panels of nine subjects) received two different single oral doses of brivaracetam (10-1400 mg) and one dose of placebo during three periods of a randomized, double-blind, placebo-controlled study. The effect of food on its pharmacokinetics was assessed using a standard two-way crossover design in a further eight subjects who received two single oral doses of brivaracetam (150 mg) in the fasting state and after a high fat meal.
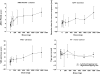
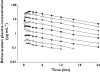
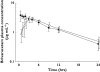
Results: Adverse events, none of which were serious, were mostly CNS-related and included somnolence, dizziness, and decreased attention, alertness, and motor control. Their incidence, severity and duration were dose-related. The maximum tolerated dose was established to be 1000 mg. Severe somnolence lasting 1 day occurred in one subject following 1400 mg. Brivaracetam was rapidly absorbed under fasting conditions, with a median t(max) of approximately 1 h. C(max) was dose-proportional from 10 to 1400 mg, whereas AUC deviated from dose linearity above 600 mg. A high-fat meal had no effect on AUC (point estimate 0.99, 90%CI: 0.92-1.07) but delayed t(max) (3 h) and decreased C(max) (point estimate 0.72, 90%CI: 0.66-0.79).
Conclusions: Brivaracetam was well tolerated after increasing single doses that represent up to several times the expected therapeutic dose. Brivaracetam was found to have desirable pharmacokinetic properties. The most common adverse events were somnolence and dizziness.
Figures

References
-
- Zona C, Pieri M, Klitgaard H, Margineanu D-G. ucb 34714, a new pyrrolidone derivative, inhibits Na+ currents in rat cortical neurons in culture. Epilepsia. 2004;45(Suppl 7):146.
-
- Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, Frycia AM, Moureau FG, Klitgaard HV, Gillard MR, Fuks B, Michel P. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–49. - PubMed
-
- Matagne A, Kenda B, Michel P, Klitgaard H. ucb 34714, a new pyrrolidone derivative, suppresses seizures epileptogenesis in animal models of chronic epilepsy in vivo. Epilepsia. 2003;44(Suppl 8):53–4.
-
- Lamberty Y, Ardid D, Eschalier A, Alloui A, Matagne A, Kenda B, Michel P, Klitgaard H. A new pyrrolidone derivative, ucb 34714, is effective in neuropathic pain models in rats: comparison with gabapentin. J Pain. 2003;4(Suppl 1):53.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

