Protein binding of cefazolin is saturable in vivo both between and within patients
- PMID: 17223858
- PMCID: PMC2000586
- DOI: 10.1111/j.1365-2125.2006.02827.x
Protein binding of cefazolin is saturable in vivo both between and within patients
Abstract
Aims: The aims of the study were a) to determine if there is evidence of saturable protein binding of cefazolin in plasma across the range of concentrations achieved clinically (between patient variability) and b) to investigate whether saturable protein binding is also evident from trough and peak concentrations in the same patient (within patient variability).
Methods: Unbound and total plasma concentrations were measured in patients who were treated with cefazolin intravenously by continuous infusion or intermittent injection. In study (i) single random samples were taken from one series of patients. In study (ii) paired samples (troughs and peaks) were taken from a second series of patients.
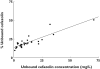
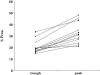
Results: Thirty-one patients were included in study (i). Linear regression analysis of the percentage unbound vs. unbound plasma concentrations revealed a slope significantly different from zero, suggesting saturable protein binding. Mean values for percentage unbound ranged from 9% at low concentrations (8.5 mg l(-1)) to 51% at high concentrations (140 mg l(-1)). Twelve patients were investigated in study (ii). Values for protein binding ranged from 85% at low concentrations (2.7 mg l(-1)) to 52% at high concentrations (200.3 mg l(-1)). The percentage unbound was significantly higher (P < 0.0001) at high (peak) concentrations than at lower (trough) concentrations, confirming saturable protein binding.
Conclusions: The protein binding of cefazolin is saturable in vivo in humans, both between and within patients.
Figures
Similar articles
-
Population pharmacokinetic modelling of total and unbound cefazolin plasma concentrations as a guide for dosing in preterm and term neonates.J Antimicrob Chemother. 2014 May;69(5):1330-8. doi: 10.1093/jac/dkt527. Epub 2014 Feb 2. J Antimicrob Chemother. 2014. PMID: 24492261
-
Effects of cardiopulmonary bypass on the disposition of cefazolin in patients undergoing cardiothoracic surgery.Pharmacol Res Perspect. 2018 Nov 5;6(6):e00440. doi: 10.1002/prp2.440. eCollection 2018 Dec. Pharmacol Res Perspect. 2018. PMID: 30410768 Free PMC article.
-
Cefazolin plasma protein binding saturability during pregnancy.Methods Find Exp Clin Pharmacol. 2009 Jan-Feb;31(1):25-8. doi: 10.1358/mf.2009.31.1.1338413. Methods Find Exp Clin Pharmacol. 2009. PMID: 19357795 Clinical Trial.
-
Cefazolin bolus and continuous administration for elective cardiac surgery: improved pharmacokinetic and pharmacodynamic parameters.J Thorac Cardiovasc Surg. 2010 Aug;140(2):471-5. doi: 10.1016/j.jtcvs.2010.03.038. Epub 2010 Jun 8. J Thorac Cardiovasc Surg. 2010. PMID: 20570290 Clinical Trial.
-
Cefazolin plasma protein binding and its covariates in neonates.Eur J Clin Microbiol Infect Dis. 2012 Dec;31(12):3359-65. doi: 10.1007/s10096-012-1703-x. Epub 2012 Jul 26. Eur J Clin Microbiol Infect Dis. 2012. PMID: 22833246
Cited by
-
Rational dosage regimens for cephalothin and cefazolin using pharmacokinetics and pharmacodynamics analysis in healthy horses.Equine Vet J. 2021 Nov;53(6):1239-1249. doi: 10.1111/evj.13406. Epub 2021 Jan 21. Equine Vet J. 2021. PMID: 33341979 Free PMC article.
-
Addressing the Accuracy of Plasma Protein Binding Measurement for Highly Bound Compounds Using the Dilution Method.AAPS J. 2022 Dec 5;25(1):7. doi: 10.1208/s12248-022-00774-2. AAPS J. 2022. PMID: 36471200
-
Population Pharmacokinetic Study of Cefazolin Dosage Adaptation in Bacteremia and Infective Endocarditis Based on a Nomogram.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00806-19. doi: 10.1128/AAC.00806-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31307987 Free PMC article.
-
Evaluation of the need for dosing adaptations in obese patients for surgical antibiotic prophylaxis: a model-based analysis of cefazolin pharmacokinetics.Br J Anaesth. 2025 Apr;134(4):1041-1049. doi: 10.1016/j.bja.2024.11.044. Epub 2025 Feb 1. Br J Anaesth. 2025. PMID: 39894750 Free PMC article.
-
The Impact of Drug Properties and Severity of Obesity on Renal Drug Clearance Through Glomerular Filtration and Active Tubular Secretion: A Systematic Analysis Using PBPK Modeling.Pharm Res. 2025 Jul;42(7):1079-1088. doi: 10.1007/s11095-025-03885-5. Epub 2025 Jun 27. Pharm Res. 2025. PMID: 40571872 Free PMC article.
References
-
- Grayson ML, Silvers J, Turnbridge J. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Australia. 1995;162:249–53. - PubMed
-
- Kirby WMM, Regamey C. Pharmacokinetics of cefazolin compared with other cephalosporins. J Infect Dis. 1973;128:S341–S346. - PubMed
-
- Nightingale CH, Greene DS, Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975;64:1900–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical