Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes
- PMID: 17225870
- PMCID: PMC1770007
- DOI: 10.2119/2006–00055.Toth-Zsamboki
Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes
Abstract
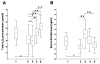
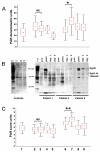
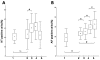
Reactive free radical and oxidant production leads to DNA damage during myocardial ischemia/reperfusion. Consequent overactivation of poly(ADP-ribose) polymerase (PARP) promotes cellular energy deficit and necrosis. We hypothesized that PARP is activated in circulating leukocytes in patients with myocardial infarction and reperfusion during primary percutaneous coronary intervention (PCI). In 15 patients with ST segment elevation acute myocardial infarction, before and after primary PCI and 24 and 96 h later, we determined serum hydrogen peroxide concentrations, plasma levels of the oxidative DNA adduct 8-hydroxy-2'-deoxyguanosine (8OHdG), tyrosine nitration, PARP activation, and translocation of apoptosis-inducing factor (AIF) in circulating leukocytes. Plasma 8OHdG levels and leukocyte tyrosine nitration were rapidly increased by PCI. Similarly, poly(ADP-ribose) content of the leukocytes increased in cells isolated just after PCI, indicating immediate PARP activation triggered by reperfusion of the myocardium. In contrast, serum hydrogen peroxide concentrations and the translocation of AIF gradually increased over time and were most pronounced at 96 h. Reperfusion-related oxidative/nitrosative stress triggers DNA damage, which leads to PARP activation in circulating leukocytes. Translocation of AIF and lipid peroxidation occurs at a later stage. These results represent the first direct demonstration of PARP activation in human myocardial infarction. Future work is required to test whether pharmacological inhibition of PARP may offer myocardial protection during primary PCI.
Figures

Similar articles
-
Activation of poly(ADP-ribose) polymerase in circulating leukocytes during myocardial infarction.Shock. 2004 Mar;21(3):230-4. doi: 10.1097/01.shk.0000110621.42625.10. Shock. 2004. PMID: 14770035
-
Increased poly(ADP-ribosyl)ation in peripheral leukocytes and the reperfused myocardium tissue of rats with ischemia/reperfusion injury: prevention by 3-aminobenzamide treatment.Shock. 2012 May;37(5):492-500. doi: 10.1097/SHK.0b013e31824989d7. Shock. 2012. PMID: 22266967
-
Suppression of poly (ADP-ribose) polymerase activation by 3-aminobenzamide in a rat model of myocardial infarction: long-term morphological and functional consequences.Br J Pharmacol. 2001 Aug;133(8):1424-30. doi: 10.1038/sj.bjp.0704185. Br J Pharmacol. 2001. PMID: 11498530 Free PMC article.
-
Poly(ADP-ribose) polymerase activation in the reperfused myocardium.Cardiovasc Res. 2004 Feb 15;61(3):471-80. doi: 10.1016/j.cardiores.2003.09.029. Cardiovasc Res. 2004. PMID: 14962478 Review.
-
Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion.Free Radic Biol Med. 2005 Jul 1;39(1):81-90. doi: 10.1016/j.freeradbiomed.2005.03.021. Epub 2005 Apr 8. Free Radic Biol Med. 2005. PMID: 15925280 Review.
Cited by
-
The Angiotensin-converting enzyme inhibitor captopril inhibits poly(adp-ribose) polymerase activation and exerts beneficial effects in an ovine model of burn and smoke injury.Shock. 2011 Oct;36(4):402-9. doi: 10.1097/SHK.0b013e318228f614. Shock. 2011. PMID: 21701415 Free PMC article.
-
Modulation of poly(ADP-ribose) polymerase-1 (PARP-1)-mediated oxidative cell injury by ring finger protein 146 (RNF146) in cardiac myocytes.Mol Med. 2014 Jul 31;20(1):313-28. doi: 10.2119/molmed.2014.00102. Mol Med. 2014. PMID: 24842055 Free PMC article.
-
gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury.Free Radic Biol Med. 2008 Aug 15;45(4):425-33. doi: 10.1016/j.freeradbiomed.2008.04.037. Epub 2008 May 3. Free Radic Biol Med. 2008. PMID: 18503777 Free PMC article.
-
Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors.Cardiovasc Drug Rev. 2007 Fall;25(3):235-60. doi: 10.1111/j.1527-3466.2007.00018.x. Cardiovasc Drug Rev. 2007. PMID: 17919258 Free PMC article. Review.
-
The Protective Role of Molecular Hydrogen in Ischemia/Reperfusion Injury.Int J Mol Sci. 2024 Jul 18;25(14):7884. doi: 10.3390/ijms25147884. Int J Mol Sci. 2024. PMID: 39063126 Free PMC article. Review.
References
-
- DeWood MA, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897–902. - PubMed
-
- Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–90. - PubMed
-
- Piper HM, Meuter K, Schafer C. Cellular mechanism of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:644–8. - PubMed
-
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
