Planar cell polarity signaling in vertebrates
- PMID: 17226800
- PMCID: PMC4158832
- DOI: 10.1002/bies.20526
Planar cell polarity signaling in vertebrates
Abstract
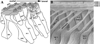
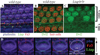
Planar cell polarity (PCP) refers to the polarization of a field of cells within the plane of a cell sheet. This form of polarization is required for diverse cellular processes in vertebrates, including convergent extension (CE), the establishment of PCP in epithelial tissues and ciliogenesis. Perhaps the most distinct example of vertebrate PCP is the uniform orientation of stereociliary bundles at the apices of sensory hair cells in the mammalian auditory sensory organ. The establishment of PCP in the mammalian cochlea occurs concurrently with CE in this ciliated epithelium, therefore linking three cellular processes regulated by the vertebrate PCP pathway in the same tissue and emerging as a model system for dissecting PCP signaling. This review summarizes the morphogenesis of this model system to assist the interpretation of the emerging data and proposes molecular mechanisms underlying PCP signaling in vertebrates.
Copyright 2007 Wiley Periodicals, Inc.
Figures


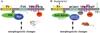

References
-
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. - PubMed
-
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. - PubMed
-
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. - PubMed
-
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. - PubMed
-
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

