Glutathione transferase omega 1 catalyzes the reduction of S-(phenacyl)glutathiones to acetophenones
- PMID: 17226937
- PMCID: PMC2562259
- DOI: 10.1021/tx600305y
Glutathione transferase omega 1 catalyzes the reduction of S-(phenacyl)glutathiones to acetophenones
Abstract
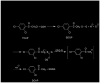
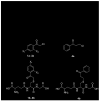
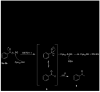
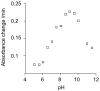
S-(Phenacyl)glutathione reductase (SPG-R) plays a significant role in the biotransformation of reactive alpha-haloketones to nontoxic acetophenones. Comparison of the apparent subunit size, amino acid composition, and catalysis of the reduction of S-(phenacyl)glutathiones indicated that a previously described rat SPG-R (Kitada, M., McLenithan, J. C., and Anders, M. W. (1985) J. Biol. Chem. 260, 11749-11754) is homologous to the omega-class glutathione transferase GSTO1-1. The available data show that the SPG-R reaction is catalyzed by GSTO1-1 and not by other GSTs, including the closely related GSTO2-2 isoenzyme. In the proposed reaction mechanism, the active-site cysteine residue of GSTO1-1 reacts with the S-(phenacyl)glutathione substrate to give an acetophenone and a mixed disulfide with the active-site cysteine; a second thiol substrate (e.g., glutathione or 2-mercaptoethanol) reacts with the active-site disulfide to regenerate the catalytically active enzyme and to form a mixed disulfide. A new spectrophotometric assay was developed that allows the rapid determination of SPG-R activity and specific measurement of GSTO1-1 in the presence of other GSTs. This is the first specific reaction attributed to GSTO1-1, and these results demonstrate the catalytic diversity of GSTO1-1, which, in addition to SPG-R activity, catalyzes the reduction of dehydroascorbate and monomethylarsonate(V) and also possesses thioltransferase and GST activity.
Figures

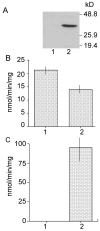
References
-
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. - PubMed
-
- Board PG, Coggan M, Chelvanayagam G, Easteal S, Jermiin LS, Schulte GK, Danley DE, Hoth LR, Griffor MC, Kamath AV, Rosner MH, Chrunyk BA, Perregaux DE, Gabel CA, Geoghegan KF, Pandit J. Identification, Characterization and Crystal structure of the Omega Class Glutathione Transferases. J Biol Chem. 2000;275:24798–24806. - PubMed
-
- Whitbread AK, Masoumi A, Tetlow N, Schmuck E, Coggan M, Board PG. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005;401:78–99. - PubMed
-
- Whitbread AK, Tetlow N, Eyre HJ, Sutherland GR, Board PG. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics. 2003;13:131–144. - PubMed
-
- Zakharyan RA, Sampayo-Reyes A, Healy SM, Tsaprailis G, Board PG, Liebler DC, Aposhian HV. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem Res Toxicol. 2001;14:1051–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

