Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d'Ivoire
- PMID: 17227132
- PMCID: PMC1769413
- DOI: 10.1371/journal.pmed.0040017
Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d'Ivoire
Abstract
Background: Little is known about the long-term safety of infant feeding interventions aimed at reducing breast milk HIV transmission in Africa.
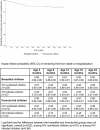
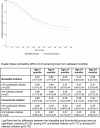
Methods and findings: In 2001-2005, HIV-infected pregnant women having received in Abidjan, Côte d'Ivoire, a peripartum antiretroviral prophylaxis were presented antenatally with infant feeding interventions: either artificial feeding, or exclusive breast-feeding and then early cessation from 4 mo of age. Nutritional counseling and clinical management were provided for 2 y. Breast-milk substitutes were provided for free. The primary outcome was the occurrence of adverse health outcomes in children, defined as validated morbid events (diarrhea, acute respiratory infections, or malnutrition) or severe events (hospitalization or death). Hazards ratios to compare formula-fed versus short-term breast-fed (reference) children were adjusted for confounders (baseline covariates and pediatric HIV status as a time-dependant covariate). The 18-mo mortality rates were also compared to those observed in the Ditrame historical trial, which was conducted at the same sites in 1995-1998, and in which long-term breast-feeding was practiced in the absence of any specific infant feeding intervention. Of the 557 live-born children, 262 (47%) were breast-fed for a median of 4 mo, whereas 295 were formula-fed. Over the 2-y follow-up period, 37% of the formula-fed and 34% of the short-term breast-fed children remained free from any adverse health outcome (adjusted hazard ratio [HR]: 1.10; 95% confidence interval [CI], 0.87-1.38; p = 0.43). The 2-y probability of presenting with a severe event was the same among formula-fed (14%) and short-term breast-fed children (15%) (adjusted HR, 1.19; 95% CI, 0.75-1.91; p = 0.44). An overall 18-mo probability of survival of 96% was observed among both HIV-uninfected short-term and formula-fed children, which was similar to the 95% probability observed in the long-term breast-fed ones of the Ditrame trial.
Conclusions: The 2-y rates of adverse health outcomes were similar among short-term breast-fed and formula-fed children. Mortality rates did not differ significantly between these two groups and, after adjustment for pediatric HIV status, were similar to those observed among long-term breast-fed children. Given appropriate nutritional counseling and care, access to clean water, and a supply of breast-milk substitutes, these alternatives to prolonged breast-feeding can be safe interventions to prevent mother-to-child transmission of HIV in urban African settings.
Conflict of interest statement
Figures

Comment in
-
When is replacement feeding safe for infants of HIV-infected women?PLoS Med. 2007 Jan;4(1):e30. doi: 10.1371/journal.pmed.0040030. PLoS Med. 2007. PMID: 17227137 Free PMC article. No abstract available.
-
Children born to HIV-infected mothers in Côte d'Ivoire: methodological clarifications needed.PLoS Med. 2007 Mar 27;4(3):e140. doi: 10.1371/journal.pmed.0040140. PLoS Med. 2007. PMID: 17388678 Free PMC article. No abstract available.
References
-
- Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, et al. Breastfeeding and HIV International Transmission Study Group (2004) Late postnatal transmission of HIV-1 in breast-fed children: An individual patient data meta-analysis. J Infect Dis. 189:2154–2166. - PubMed
-
- Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA. 2000;283:1167–1174. - PubMed
-
- WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: A pooled analysis. Lancet. 2000;355:451–455. - PubMed
-
- HIV and infant feeding: Guidelines for decision-makers/UNICEF, UNAIDS, WHO, UNFPA. Geneva: World Health Organization; 2003. Available: http://www.who.int/child-adolescent-health/New_Publications/NUTRITION/HI.... Accessed 21 November 2006.

