Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons
- PMID: 17227870
- PMCID: PMC1783140
- DOI: 10.1073/pnas.0605208103
Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons
Abstract
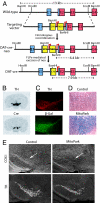
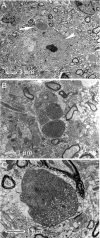
Mitochondrial dysfunction is implicated in the pathophysiology of Parkinson's disease (PD), a common age-associated neurodegenerative disease characterized by intraneuronal inclusions (Lewy bodies) and progressive degeneration of the nigrostriatal dopamine (DA) system. It has recently been demonstrated that midbrain DA neurons of PD patients and elderly humans contain high levels of somatic mtDNA mutations, which may impair respiratory chain function. However, clinical studies have not established whether the respiratory chain deficiency is a primary abnormality leading to inclusion formation and DA neuron death, or whether generalized metabolic abnormalities within the degenerating DA neurons cause secondary damage to mitochondria. We have used a reverse genetic approach to investigate this question and created conditional knockout mice (termed MitoPark mice), with disruption of the gene for mitochondrial transcription factor A (Tfam) in DA neurons. The knockout mice have reduced mtDNA expression and respiratory chain deficiency in midbrain DA neurons, which, in turn, leads to a parkinsonism phenotype with adult onset of slowly progressive impairment of motor function accompanied by formation of intraneuronal inclusions and dopamine nerve cell death. Confocal and electron microscopy show that the inclusions contain both mitochondrial protein and membrane components. These experiments demonstrate that respiratory chain dysfunction in DA neurons may be of pathophysiological importance in PD.
Conflict of interest statement
Conflict of interest statement: N.-G.L., L.O., and M.I.E. are coowners of a company owning commercial rights to the MitoPark mice.
Figures
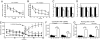

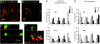
References
-
- Gaspari M, Larsson NG, Gustafsson CM. Biochim Biophys Acta. 2004;1659:148–152. - PubMed
-
- Dauer W, Przedborski S. Neuron. 2003;39:889–909. - PubMed
-
- Shen J, Cookson MR. Neuron. 2004;43:301–304. - PubMed
-
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Nature. 2006;441:1157–1161. - PubMed
-
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Nature. 2006;441:1162–1166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

