Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells
- PMID: 17227908
- PMCID: PMC2118428
- DOI: 10.1084/jem.20061115
Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells
Erratum in
- J Exp Med. 2007 Jul 9;204(7):1729
Abstract
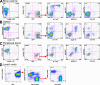
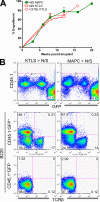
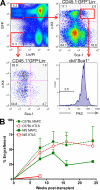
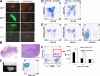
For decades, in vitro expansion of transplantable hematopoietic stem cells (HSCs) has been an elusive goal. Here, we demonstrate that multipotent adult progenitor cells (MAPCs), isolated from green fluorescent protein (GFP)-transgenic mice and expanded in vitro for >40-80 population doublings, are capable of multilineage hematopoietic engraftment of immunodeficient mice. Among MAPC-derived GFP+CD45.2+ cells in the bone marrow of engrafted mice, HSCs were present that could radioprotect and reconstitute multilineage hematopoiesis in secondary and tertiary recipients, as well as myeloid and lymphoid hematopoietic progenitor subsets and functional GFP+ MAPC-derived lymphocytes that were functional. Although hematopoietic contribution by MAPCs was comparable to control KTLS HSCs, approximately 10(3)-fold more MAPCs were required for efficient engraftment. Because GFP+ host-derived CD45.1+ cells were not observed, fusion is not likely to account for the generation of HSCs by MAPCs.
Figures
Similar articles
-
Multilineage engraftment in NOD/LtSz-scid/scid mice from mobilized human CD34+ peripheral blood progenitor cells.Biol Blood Marrow Transplant. 1997 Nov;3(5):236-46. Biol Blood Marrow Transplant. 1997. PMID: 9450918
-
Cord blood CD34+ cells expanded on Wharton's jelly multipotent mesenchymal stromal cells improve the hematopoietic engraftment in NOD/SCID mice.Eur J Haematol. 2014 Nov;93(5):384-91. doi: 10.1111/ejh.12363. Epub 2014 May 26. Eur J Haematol. 2014. PMID: 24797266
-
Stem Cell Defect in Ubiquitin-Green Fluorescent Protein Mice Facilitates Engraftment of Lymphoid-Primed Hematopoietic Stem Cells.Stem Cells. 2018 Aug;36(8):1237-1248. doi: 10.1002/stem.2828. Epub 2018 Apr 15. Stem Cells. 2018. PMID: 29603838
-
Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors.Immunol Rev. 2010 Nov;238(1):37-46. doi: 10.1111/j.1600-065X.2010.00963.x. Immunol Rev. 2010. PMID: 20969583 Free PMC article. Review.
-
Engineering humanized mice for improved hematopoietic reconstitution.Cell Mol Immunol. 2012 May;9(3):215-24. doi: 10.1038/cmi.2012.6. Epub 2012 Mar 19. Cell Mol Immunol. 2012. PMID: 22425741 Free PMC article. Review.
Cited by
-
Bone marrow mesenchymal stem cells: historical overview and concepts.Hum Gene Ther. 2010 Sep;21(9):1045-56. doi: 10.1089/hum.2010.115. Hum Gene Ther. 2010. PMID: 20565251 Free PMC article. Review.
-
An irradiated marrow niche reveals a small noncollagenous protein mediator of homing, dermatopontin.Blood Adv. 2021 Sep 28;5(18):3609-3622. doi: 10.1182/bloodadvances.2021004475. Blood Adv. 2021. PMID: 34448828 Free PMC article.
-
Evaluating in vivo efficacy - toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells.J Immunother Cancer. 2019 Mar 12;7(1):69. doi: 10.1186/s40425-019-0558-4. J Immunother Cancer. 2019. PMID: 30871629 Free PMC article.
-
De novo formed satellite DNA-based mammalian artificial chromosomes and their possible applications.Chromosome Res. 2015 Feb;23(1):143-57. doi: 10.1007/s10577-014-9458-0. Chromosome Res. 2015. PMID: 25596828 Review.
-
A Review of the Evidence for Tryptophan and the Kynurenine Pathway as a Regulator of Stem Cell Niches in Health and Disease.Int J Tryptophan Res. 2024 May 15;17:11786469241248287. doi: 10.1177/11786469241248287. eCollection 2024. Int J Tryptophan Res. 2024. PMID: 38757094 Free PMC article. Review.
References
-
- Morrison, S.J., and I.L. Weissman. 1994. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1:661–673. - PubMed
-
- Sorrentino, B.P. 2004. Clinical strategies for expansion of haematopoietic stem cells. Nat. Rev. Immunol. 4:878–888. - PubMed
-
- Kyba, M., R.C. Perlingeiro, and G.Q. Daley. 2002. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 109:29–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL63452/HL/NHLBI NIH HHS/United States
- R01 HL073794/HL/NHLBI NIH HHS/United States
- R01 HL052952/HL/NHLBI NIH HHS/United States
- T15 HL076653/HL/NHLBI NIH HHS/United States
- HL073794/HL/NHLBI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- T32AR07612/AR/NIAMS NIH HHS/United States
- P01 AI 056299/AI/NIAID NIH HHS/United States
- HL058770/HL/NHLBI NIH HHS/United States
- T32 AR007612/AR/NIAMS NIH HHS/United States
- HL49997/HL/NHLBI NIH HHS/United States
- DK5829/DK/NIDDK NIH HHS/United States
- HL52952/HL/NHLBI NIH HHS/United States
- T32 AR050938/AR/NIAMS NIH HHS/United States
- R01 HL063452/HL/NHLBI NIH HHS/United States
- R01 AI047457/AI/NIAID NIH HHS/United States
- HL71228/HL/NHLBI NIH HHS/United States
- AI047457/AI/NIAID NIH HHS/United States
- HL076653/HL/NHLBI NIH HHS/United States
- R01 HL049997/HL/NHLBI NIH HHS/United States
- R01 HL058770/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

