The antiinflammatory activity of IgG: the intravenous IgG paradox
- PMID: 17227911
- PMCID: PMC2118416
- DOI: 10.1084/jem.20061788
The antiinflammatory activity of IgG: the intravenous IgG paradox
Abstract
How high doses of intravenous IgG (IVIG) suppress autoimmune diseases remains unresolved. We have recently shown that the antiinflammatory activity of IVIG can be attributed to a minor species of IgGs that is modified with terminal sialic acids on their Fc-linked glycans. Here we propose that these Fc-sialylated IgGs engage a unique receptor on macrophages that, in turn, leads to the upregulation of an inhibitory Fcgamma receptor (FcgammaR), thereby protecting against autoantibody-mediated pathology.
Figures
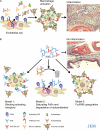

References
-
- Ravetch, J.V. 2003. Fc receptors. In Fundamental Immunology. W.E. Paul, editor. Lippincott-Raven, Philadelphia, PA. 685–700.
-
- Casadevall, A., E. Dadachova, and L.A. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695–703. - PubMed
-
- Toubi, E., and A. Etzioni. 2005. Intravenous immunoglobulin in immunodeficiency states: state of the art. Clin. Rev. Allergy Immunol. 29:167–172. - PubMed
-
- Imbach, P., S. Barandun, V. d'Apuzzo, C. Baumgartner, A. Hirt, A. Morell, E. Rossi, M. Schoni, M. Vest, and H.P. Wagner. 1981. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1:1228–1231. - PubMed
-
- Viard, I., P. Wehrli, R. Bullani, P. Schneider, N. Holler, D. Salomon, T. Hunziker, J.H. Saurat, J. Tschopp, and L.E. French. 1998. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 282:490–493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

