Mechanism of thioamide drug action against tuberculosis and leprosy
- PMID: 17227913
- PMCID: PMC2118422
- DOI: 10.1084/jem.20062100
Mechanism of thioamide drug action against tuberculosis and leprosy
Abstract
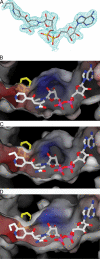
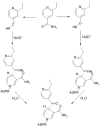
Thioamide drugs, ethionamide (ETH) and prothionamide (PTH), are clinically effective in the treatment of Mycobacterium tuberculosis, M. leprae, and M. avium complex infections. Although generally considered second-line drugs for tuberculosis, their use has increased considerably as the number of multidrug resistant and extensively drug resistant tuberculosis cases continues to rise. Despite the widespread use of thioamide drugs to treat tuberculosis and leprosy, their precise mechanisms of action remain unknown. Using a cell-based activation method, we now have definitive evidence that both thioamides form covalent adducts with nicotinamide adenine dinucleotide (NAD) and that these adducts are tight-binding inhibitors of M. tuberculosis and M. leprae InhA. The crystal structures of the inhibited M. leprae and M. tuberculosis InhA complexes provide the molecular details of target-drug interactions. The purified ETH-NAD and PTH-NAD adducts both showed nanomolar Kis against M. tuberculosis and M. leprae InhA. Knowledge of the precise structures and mechanisms of action of these drugs provides insights into designing new drugs that can overcome drug resistance.
Figures


Similar articles
-
Bacterial Genome-Wide Association Identifies Novel Factors That Contribute to Ethionamide and Prothionamide Susceptibility in Mycobacterium tuberculosis.mBio. 2019 Apr 23;10(2):e00616-19. doi: 10.1128/mBio.00616-19. mBio. 2019. PMID: 31015328 Free PMC article.
-
Drugs and drug development for chemotherapy of leprosy and tuberculosis, including M. avium infections.Trop Med Parasitol. 1990 Sep;41(3):341-3. Trop Med Parasitol. 1990. PMID: 2255857 Review. No abstract available.
-
Ethionamide biomimetic activation and an unprecedented mechanism for its conversion into active and non-active metabolites.Org Biomol Chem. 2016 Sep 21;14(37):8848-8858. doi: 10.1039/c6ob01561a. Org Biomol Chem. 2016. PMID: 27714216
-
Prothionamide and prothionamide-S-oxide in experimental leprosy.Int J Lepr Other Mycobact Dis. 1981 Sep;49(3):302-6. Int J Lepr Other Mycobact Dis. 1981. PMID: 6459296 No abstract available.
-
A review of the use of ethionamide and prothionamide in childhood tuberculosis.Tuberculosis (Edinb). 2016 Mar;97:126-36. doi: 10.1016/j.tube.2015.09.007. Epub 2015 Oct 19. Tuberculosis (Edinb). 2016. PMID: 26586647 Review.
Cited by
-
Implications of Fragment-Based Drug Discovery in Tuberculosis and HIV.Pharmaceuticals (Basel). 2022 Nov 15;15(11):1415. doi: 10.3390/ph15111415. Pharmaceuticals (Basel). 2022. PMID: 36422545 Free PMC article. Review.
-
Wollamide Cyclic Hexapeptides Synergize with Established and New Tuberculosis Antibiotics in Targeting Mycobacterium tuberculosis.Microbiol Spectr. 2023 Aug 17;11(4):e0046523. doi: 10.1128/spectrum.00465-23. Epub 2023 Jun 8. Microbiol Spectr. 2023. PMID: 37289062 Free PMC article.
-
A Coumarin-Based Analogue of Thiacetazone as Dual Covalent Inhibitor and Potential Fluorescent Label of HadA in Mycobacterium tuberculosis.ACS Infect Dis. 2021 Mar 12;7(3):552-565. doi: 10.1021/acsinfecdis.0c00325. Epub 2021 Feb 22. ACS Infect Dis. 2021. PMID: 33617235 Free PMC article.
-
Revolutionizing tuberculosis treatment: Breakthroughs, challenges, and hope on the horizon.Acta Pharm Sin B. 2025 Mar;15(3):1311-1332. doi: 10.1016/j.apsb.2025.01.023. Epub 2025 Jan 31. Acta Pharm Sin B. 2025. PMID: 40370552 Free PMC article. Review.
-
Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria.PLoS One. 2007 Dec 19;2(12):e1343. doi: 10.1371/journal.pone.0001343. PLoS One. 2007. PMID: 18094751 Free PMC article.
References
-
- Fajardo, T.T., R.S. Guinto, R.V. Cellona, R.M. Abalos, E.C. Dela Cruz, and R.H. Gelber. 2006. A clinical trial of ethionamide and prothionamide for treatment of lepromatous leprosy. Am. J. Trop. Med. Hyg. 74:457–461. - PubMed
-
- Centers for Disease Control and Prevention. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs–worldwide, 2000-2004. MMWR Morb. Mortal. Wkly. Rep. 55:301–305. - PubMed
-
- Crofton, J., P. Chaulet, D. Maher, J. Grosset, W. Harris, H. Norman, M. Iseman, and B. Watt. 1997. Guidelines for the Management of Multidrug-Resistant Tuberculosis. World Health Organization: Geneva, Switzerland.
-
- Katoch, K., M. Natarajan, A.S. Bhatia, and V.S. Yadav. 1992. Treatment of paucibacillary leprosy with a regimen containing rifampicin, dapsone and prothionamide. Indian J. Lepr. 64:303–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

