The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes
- PMID: 17227916
- PMCID: PMC2154355
- DOI: 10.1085/jgp.200609612
The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes
Abstract
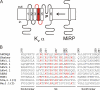
Voltage-gated potassium (Kv) channels extend their functional repertoire by coassembling with MinK-related peptides (MiRPs). MinK slows the activation of channels formed with KCNQ1 alpha subunits to generate the voltage-dependent I(Ks) channel in human heart; MiRP1 and MiRP2 remove the voltage dependence of KCNQ1 to generate potassium "leak" currents in gastrointestinal epithelia. Other Kv alpha subunits interact with MiRP1 and MiRP2 but without loss of voltage dependence; the mechanism for this disparity is unknown. Here, sequence alignments revealed that the voltage-sensing S4 domain of KCNQ1 bears lower net charge (+3) than that of any other eukaryotic voltage-gated ion channel. We therefore examined the role of KCNQ1 S4 charges in channel activation using alanine-scanning mutagenesis and two-electrode voltage clamp. Alanine replacement of R231, at the N-terminal side of S4, produced constitutive activation in homomeric KCNQ1 channels, a phenomenon not observed with previous single amino acid substitutions in S4 of other channels. Homomeric KCNQ4 channels were also made constitutively active by mutagenesis to mimic the S4 charge balance of R231A-KCNQ1. Loss of single S4 charges at positions R231 or R237 produced constitutively active MinK-KCNQ1 channels and increased the constitutively active component of MiRP2-KCNQ1 currents. Charge addition to the CO2H-terminal half of S4 eliminated constitutive activation in MiRP2-KCNQ1 channels, whereas removal of homologous charges from KCNQ4 S4 produced constitutively active MiRP2-KCNQ4 channels. The results demonstrate that the unique S4 charge paucity of KCNQ1 facilitates its unique conversion to a leak channel by ancillary subunits such as MiRP2.
Figures
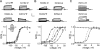
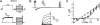
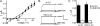



Similar articles
-
Interaction of KCNE subunits with the KCNQ1 K+ channel pore.J Physiol. 2006 Feb 1;570(Pt 3):455-67. doi: 10.1113/jphysiol.2005.100644. Epub 2005 Nov 24. J Physiol. 2006. PMID: 16308347 Free PMC article.
-
A shared mechanism for lipid- and beta-subunit-coordinated stabilization of the activated K+ channel voltage sensor.FASEB J. 2010 May;24(5):1518-24. doi: 10.1096/fj.09-145219. Epub 2009 Dec 29. FASEB J. 2010. PMID: 20040519 Free PMC article.
-
KCNE3 acts by promoting voltage sensor activation in KCNQ1.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):E7286-92. doi: 10.1073/pnas.1516238112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2015. PMID: 26668384 Free PMC article.
-
Impact of ancillary subunits on ventricular repolarization.J Electrocardiol. 2007 Nov-Dec;40(6 Suppl):S42-6. doi: 10.1016/j.jelectrocard.2007.05.021. J Electrocardiol. 2007. PMID: 17993327 Free PMC article. Review.
-
The impact of ancillary subunits on small-molecule interactions with voltage-gated potassium channels.Curr Pharm Des. 2006;12(18):2285-302. doi: 10.2174/138161206777585175. Curr Pharm Des. 2006. PMID: 16787255 Review.
Cited by
-
Purinergic receptors are a key bottleneck in tumor metabolic reprogramming: The prime suspect in cancer therapeutic resistance.Front Immunol. 2022 Aug 22;13:947885. doi: 10.3389/fimmu.2022.947885. eCollection 2022. Front Immunol. 2022. PMID: 36072596 Free PMC article. Review.
-
Structural basis for K(V)7.1-KCNE(x) interactions in the I(Ks) channel complex.Heart Rhythm. 2010 May;7(5):708-13. doi: 10.1016/j.hrthm.2009.12.017. Epub 2009 Dec 24. Heart Rhythm. 2010. PMID: 20206317 Free PMC article. Review.
-
Cilantro leaf harbors a potent potassium channel-activating anticonvulsant.FASEB J. 2019 Oct;33(10):11349-11363. doi: 10.1096/fj.201900485R. Epub 2019 Jul 16. FASEB J. 2019. PMID: 31311306 Free PMC article.
-
Gating consequences of charge neutralization of arginine residues in the S4 segment of K(v)7.2, an epilepsy-linked K+ channel subunit.Biophys J. 2008 Sep;95(5):2254-64. doi: 10.1529/biophysj.107.128371. Epub 2008 May 30. Biophys J. 2008. PMID: 18515377 Free PMC article.
-
Grafting voltage and pharmacological sensitivity in potassium channels.Cell Res. 2016 Aug;26(8):935-45. doi: 10.1038/cr.2016.57. Epub 2016 May 13. Cell Res. 2016. PMID: 27174053 Free PMC article.
References
-
- Abbott, G.W., M.H. Butler, S. Bendahhou, M.C. Dalakas, L.J. Ptacek, and S.A. Goldstein. 2001. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 104:217–231. - PubMed
-
- Abbott, G.W., F. Sesti, I. Splawski, M.E. Buck, M.H. Lehmann, K.W. Timothy, M.T. Keating, and S.A. Goldstein. 1999. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 97:175–187. - PubMed
-
- Aggarwal, S.K., and R. MacKinnon. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 16:1169–1177. - PubMed
-
- Barhanin, J., F. Lesage, E. Guillemare, M. Fink, M. Lazdunski, and G. Romey. 1996. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 384:78–80. - PubMed
-
- Grahammer, F., A.W. Herling, H.J. Lang, A. Schmitt-Graff, O.H. Wittekindt, R. Nitschke, M. Bleich, J. Barhanin, and R. Warth. 2001. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 120:1363–1371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

