Importance of hydration and dynamics on the selectivity of the KcsA and NaK channels
- PMID: 17227917
- PMCID: PMC2154357
- DOI: 10.1085/jgp.200609633
Importance of hydration and dynamics on the selectivity of the KcsA and NaK channels
Erratum in
- J Gen Physiol. 2011 Dec;138(6):651
Abstract
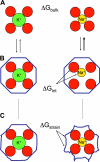
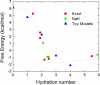
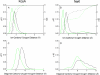
Fundamental concepts governing ion selectivity in narrow pores are reviewed and the microscopic factors responsible for the lack of selectivity of the NaK channel, which is structurally similar to the K+-selective KcsA channel, are elucidated on the basis of all-atom molecular dynamics free energy simulations. The results on NaK are contrasted and compared with previous studies of the KcsA channel. Analysis indicates that differences in hydration of the cation in the pore of NaK is at the origin of the lack of selectivity of NaK.
Figures

Similar articles
-
Absence of ion-binding affinity in the putatively inactivated low-[K+] structure of the KcsA potassium channel.Structure. 2011 Jan 12;19(1):70-9. doi: 10.1016/j.str.2010.10.008. Structure. 2011. PMID: 21220117
-
Atomic structure of a Na+- and K+-conducting channel.Nature. 2006 Mar 23;440(7083):570-4. doi: 10.1038/nature04508. Epub 2006 Feb 8. Nature. 2006. PMID: 16467789
-
Exploring the origin of the ion selectivity of the KcsA potassium channel.Proteins. 2003 Aug 15;52(3):412-26. doi: 10.1002/prot.10455. Proteins. 2003. PMID: 12866052
-
Ion selectivity in potassium channels.Biophys Chem. 2006 Dec 1;124(3):279-91. doi: 10.1016/j.bpc.2006.05.033. Epub 2006 Jun 18. Biophys Chem. 2006. PMID: 16843584 Review.
-
Structural studies of ion selectivity in tetrameric cation channels.J Gen Physiol. 2011 May;137(5):397-403. doi: 10.1085/jgp.201010546. J Gen Physiol. 2011. PMID: 21518828 Free PMC article. Review. No abstract available.
Cited by
-
Kinetics and thermodynamics of binding reactions as exemplified by anthrax toxin channel blockage with a cationic cyclodextrin derivative.Proc Natl Acad Sci U S A. 2012 Nov 6;109(45):18453-8. doi: 10.1073/pnas.1208771109. Epub 2012 Oct 24. Proc Natl Acad Sci U S A. 2012. PMID: 23100532 Free PMC article.
-
Ion binding sites and their representations by reduced models.J Phys Chem B. 2012 Jun 14;116(23):6966-79. doi: 10.1021/jp3007365. Epub 2012 Apr 30. J Phys Chem B. 2012. PMID: 22494321 Free PMC article.
-
Conduction of Na+ and K+ through the NaK channel: molecular and Brownian dynamics studies.Biophys J. 2008 Aug;95(4):1600-11. doi: 10.1529/biophysj.107.126722. Epub 2008 May 2. Biophys J. 2008. PMID: 18456826 Free PMC article.
-
Quantitative insights into the mechanism of proton conduction and selectivity for the human voltage-gated proton channel Hv1.Proc Natl Acad Sci U S A. 2024 Sep 17;121(38):e2407479121. doi: 10.1073/pnas.2407479121. Epub 2024 Sep 11. Proc Natl Acad Sci U S A. 2024. PMID: 39259593 Free PMC article.
-
K+/Na+ selectivity in K channels and valinomycin: over-coordination versus cavity-size constraints.J Mol Biol. 2008 Feb 8;376(1):13-22. doi: 10.1016/j.jmb.2007.11.059. Epub 2007 Nov 28. J Mol Biol. 2008. PMID: 18155244 Free PMC article.
References
-
- Åqvist, J. 1990. Ion water interaction potential derived from free energy perturbation simulations. J. Phys. Chem. 94:8021–8024.
-
- Åqvist, J., and V. Luzhkov. 2000. Ion permeation mechanism of the potassium channel. Nature. 404:881–884. - PubMed
-
- Asthagiri, D., L.R. Pratt, and M.E. Paulaitis. 2006. Role of fluctuations in a snug-fit mechanism of KcsA channel selectivity. J. Chem. Phys. 125:24701. - PubMed
-
- Bernèche, S., and B. Roux. 2001. Energetics of ion conduction through the K+ channel. Nature. 414:73–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

