The location of a closed channel gate in the GABAA receptor channel
- PMID: 17227918
- PMCID: PMC2154354
- DOI: 10.1085/jgp.200609639
The location of a closed channel gate in the GABAA receptor channel
Abstract
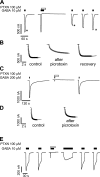
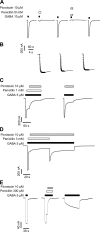
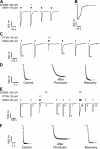
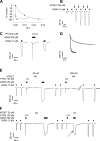
Considerable controversy surrounds the location of the closed channel gate in members of the Cys-loop receptor family of neurotransmitter-gated ion channels that includes the GABAA, glycine, acetylcholine, and 5-HT3 receptors. Cysteine-accessibility studies concluded that the gate is near the cytoplasmic end of the channel in acetylcholine and GABAA receptors but in the middle of the 5-HT3A receptor channel. Zn2+ accessibility studies in a chimeric 5-HT3-ACh receptor suggested the gate is near the channel's cytoplasmic end. In the 4-A resolution structure of the acetylcholine receptor closed state determined by cryoelectron microscopy, the narrowest region, inferred to be the gate, is in the channel's midsection from 9' to 14' but the M1-M2 loop residues at the channel's cytoplasmic end were not resolved in that structure. We used blocker trapping experiments with picrotoxin, a GABAA receptor open channel blocker, to determine whether a gate exists at a position more extracellular than the picrotoxin binding site, which is in the vicinity of alpha1Val257 (2') near the channel's cytoplasmic end. We show that picrotoxin can be trapped in the channel after removal of GABA. By using the state-dependent accessibility of engineered cysteines as reporters for the channel's structural state we infer that after GABA washout, with picrotoxin trapped in the channel, the channel appears to be in the closed state. We infer that a gate exists between the picrotoxin binding site and the channel's extracellular end, consistent with a closed channel gate in the middle of the channel. Given the homology with acetylcholine and 5-HT3 receptors there is probably a similar gate in those channels as well. This does not preclude the existence of an additional gate at a more cytoplasmic location.
Figures

References
-
- Absalom, N.L., T.M. Lewis, and P.R. Schofield. 2004. Mechanisms of channel gating of the ligand-gated ion channel superfamily inferred from protein structure. Exp. Physiol. 89:145–153. - PubMed
-
- Akabas, M.H. 2004. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int. Rev. Neurobiol. 62:1–43. - PubMed
-
- Akabas, M.H., C. Kaufmann, P. Archdeacon, and A. Karlin. 1994. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α subunit. Neuron. 13:919–927. - PubMed
-
- Amiri, S., K. Tai, O. Beckstein, P.C. Biggin, and M.S. Sansom. 2005. The α7 nicotinic acetylcholine receptor: molecular modelling, electrostatics, and energetics. Mol. Membr. Biol. 22:151–162. - PubMed
-
- Arevalo, E., D.C. Chiara, S.A. Forman, J.B. Cohen, and K.W. Miller. 2005. Gating-enhanced accessibility of hydrophobic sites within the transmembrane region of the nicotinic acetylcholine receptor's δ subunit. A time-resolved photolabeling study. J. Biol. Chem. 280:13631–13640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

