Insights into the gyrification of developing ferret brain by magnetic resonance imaging
- PMID: 17229284
- PMCID: PMC2100265
- DOI: 10.1111/j.1469-7580.2006.00674.x
Insights into the gyrification of developing ferret brain by magnetic resonance imaging
Abstract
The developmental mechanisms underlying the formation of human cortical convolutions (gyri and sulci) remain largely unknown. Genetic causes of lissencephaly (literally 'smooth brain') would imply that disorders in neuronal migration cause the loss of cortical convolutions. However, prior studies have suggested that loss of sulci and gyri can also arise from impaired proliferation, disrupted lamination and loss of intracortical connections. To gain further insight into the mechanisms underlying the formation of cortical convolutions, we examined the progressive brain development of the gyrencephalic ferret. In this study, we used magnetic resonance imaging to follow the temporal and spatial pattern of neuronal migration, proliferation and differentiation in relation to the onset and development of cortical convolutions. In this manner, we demonstrate that the onset of gyrification begins largely after completion of neuronal proliferation and migration. Gyrification occurs in a lateral to medial gradient, during the period of most rapid cerebral cortical growth. Cortical folding is also largely complete prior to myelination of the underlying cortical axons. These observations are consistent with gyrification arising secondary to cortical processes involving neuronal differentiation.
Figures
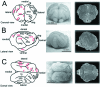



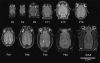


References
-
- Benveniste H, Blackband S. MR microscopy and high resolution small animal MRI: applications in neuroscience research. Prog Neurobiol. 2002;67:393–420. - PubMed
-
- Clark GD. Cerebral gyral dysplasias: molecular genetics and cell biology. Curr Opin Neurol. 2001;14:157–162. - PubMed
-
- Fox JG. Biology and Diseases of the Ferret. Baltimore, MD: Lippincott, Williams & Wilkins; 1998.
-
- Francis F, Meyer G, Fallet-Bianco C, et al. Human disorders of cortical development: from past to present. Eur J Neurosci. 2006;23:877–893. - PubMed
-
- Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210:411–417. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

