Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut
- PMID: 17229501
- PMCID: PMC1839821
- DOI: 10.1016/j.vaccine.2006.11.044
Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut
Abstract
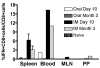
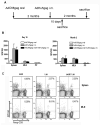
Gut-associated lymphoid tissue (GALT) is the primary replication site for HIV-1, resulting in a pronounced CD4(+) T cell loss in this tissue during primary infection. A mucosal vaccine that generates HIV-specific CD8(+) T cells in the gut could prevent the establishment of founder populations and broadcasting of virus. Here, we immunized mice orally and systemically with a chimpanzee derived adenoviral vector expressing HIV gag (AdC68gag) and measured frequencies of gag-specific interferon-gamma (IFN-gamma) producing CD8(+) T cells in the GALT. A single oral administration was inefficient at eliciting responses in the mesenteric lymph nodes and Peyer's Patches, while a single intramuscular administration elicited strong systemic and detectable mucosal responses. The gag-specific CD8(+) T cell responses were present in both acute and memory phases following intramuscular administration.
Figures
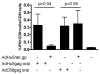
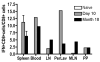
References
-
- Veazey RS, Lackner AA. HIV swiftly guts the immune system. Nat Med. 2005;11(5):469–70. - PubMed
-
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5(10):783–92. - PubMed
-
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–53. - PubMed
-
- Sharpe S, Fooks A, Lee J, Hayes K, Clegg C, Cranage M. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology. 2002;293(2):210–6. - PubMed
-
- Stevceva L, Strober W. Mucosal HIV vaccines: where are we now? Curr HIV Res. 2004;2(1):1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

