Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator
- PMID: 17229675
- PMCID: PMC2271070
- DOI: 10.1152/ajprenal.00434.2006
Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator
Abstract
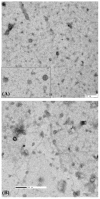
Urinary exosomes are excreted from all nephron segments and may serve as biomarkers for classifying renal diseases. Isolation of urinary exosomes by the established ultracentrifugation method has some limitations for use in a clinical laboratory. We sought a rapid and simple way to obtain urinary exosomes. We used a commercially available nanomembrane concentrator to enrich exosomes from urine by centrifugation at 3,000 g for 10-30 min. Urinary exosomal markers tumor susceptibility gene 101, aquaporin-2, neuron-specific enolase, annexin V, angiotensin-converting enzyme, and podocalyxin (PODXL) were recovered from the nanomembrane concentrator and detected by Western blotting, and typical features of urinary vesicles were found by electron microscopy. Exosomal markers were detected in as little as 0.5 ml of urine. By the nanomembrane method, exosomal proteins could be recovered from urine samples frozen at -80 degrees C or refrigerated overnight at 4 degrees C then stored at -80 degrees C. By enriching exosomes we could detect PODXL, a podocyte marker, which decreased by 71% in five male patients with focal segmental glomerulosclerosis and abundant proteinuria. We conclude that 1) use of a nanomembrane concentrator simplifies and accelerates the enrichment of urinary exosomes; and 2) the nanomembrane concentrator can concentrate exosomal proteins from clinical urine samples. This enhanced method may accelerate the translation of urinary exosomal biomarkers from bench to bedside for the diagnosis, classification, and prognostication of renal diseases.
Figures




References
-
- du Cheyron D, Daubin C, Poggioli J, Ramakers M, Houillier P, Charbonneau P, Paillard M. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis. 2003;42:497–506. - PubMed
-
- Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. - PubMed
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. - PubMed
-
- Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

