Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways
- PMID: 17229689
- PMCID: PMC1866082
- DOI: 10.1128/JVI.02192-06
Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways
Abstract
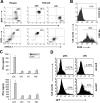
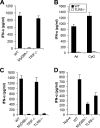
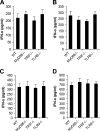
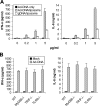
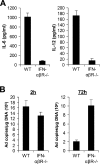
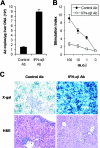
Recombinant adenoviral vectors have been widely used for gene therapy applications and as vaccine vehicles for treating infectious diseases such as human immunodeficiency virus disease. The innate immune response to adenoviruses represents the most significant hurdle in clinical application of adenoviral vectors for gene therapy, but it is an attractive feature for vaccine development. How adenovirus activates innate immunity remains largely unknown. Here we showed that adenovirus elicited innate immune response through the induction of high levels of type I interferons (IFNs) by both plasmacytoid dendritic cells (pDCs) and non-pDCs such as conventional DCs and macrophages. The innate immune recognition of adenovirus by pDCs was mediated by Toll-like receptor 9 (TLR9) and was dependent on MyD88, whereas that by non-pDCs was TLR independent through cytosolic sensing of adenoviral DNA. Furthermore, type I IFNs were pivotal in innate and adaptive immune responses to adenovirus in vivo, and type I IFN blockade diminished immune responses, resulting in more stable transgene expression and reduction of inflammation. These findings indicate that adenovirus activates innate immunity by its DNA through TLR-dependent and -independent pathways in a cell type-specific fashion, and they highlight a critical role for type I IFNs in innate and adaptive immune responses to adenoviral vectors. Our results that suggest strategies to interfere with type I IFN pathway may improve the outcome of adenovirus-mediated gene therapy, whereas approaches to activate the type I IFN pathway may enhance vaccine potency.
Figures


References
-
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Barouch, D. H., and G. J. Nabel. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16:149-156. - PubMed
-
- Basner-Tschakarjan, E., E. Gaffal, M. O'Keeffe, D. Tormo, A. Limmer, H. Wagner, H. Hochrein, and T. Tuting. 2006. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 8:1300-1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

