Increased apoptosis, p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice
- PMID: 17229834
- PMCID: PMC1783131
- DOI: 10.1073/pnas.0610619104
Increased apoptosis, p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice
Abstract
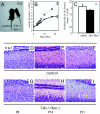
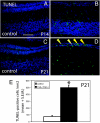
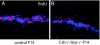
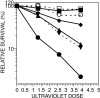
Cockayne syndrome (CS) is a rare recessive childhood-onset neurodegenerative disease, characterized by a deficiency in the DNA repair pathway of transcription-coupled nucleotide excision repair. Mice with a targeted deletion of the CSB gene (Csb-/-) exhibit a much milder ataxic phenotype than human patients. Csb-/- mice that are also deficient in global genomic repair [Csb-/-/xeroderma pigmentosum C (Xpc)-/-] are more profoundly affected, exhibiting whole-body wasting, ataxia, and neural loss by postnatal day 21. Cerebellar granule cells demonstrated high TUNEL staining indicative of apoptosis. Purkinje cells, identified by the marker calbindin, were severely depleted and, although not TUNEL-positive, displayed strong immunoreactivity for p53, indicating cellular stress. A subset of animals heterozygous for Csb and Xpc deficiencies was more mildly affected, demonstrating ataxia and Purkinje cell loss at 3 months of age. Mouse, Csb-/-, and Xpc-/- embryonic fibroblasts each exhibited increased sensitivity to UV light, which generates bulky DNA damage that is a substrate for excision repair. Whereas Csb-/-/Xpc-/- fibroblasts were more UV-sensitive than either single knockout, double-heterozygote fibroblasts had normal UV sensitivity. Csb-/- mice crossed with a strain defective in base excision repair (Ogg1) demonstrated no enhanced neurodegenerative phenotype. Complete deficiency in nucleotide excision repair therefore renders the brain profoundly sensitive to neurodegeneration in specific cell types of the cerebellum, possibly because of unrepaired endogenous DNA damage that is a substrate for nucleotide but not base excision repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nance MA, Berry SA. Am J Med Genet. 1992;42:68–84. - PubMed
-
- Leech RW, Brumback RA, Miller RH, Otsuka F, Tarone RE, Robbins JH. J Neuropathol Exp Neurol. 1985;44:507–519. - PubMed
-
- Wood RD, Mitchell M, Sgouros J, Lindahl T. Science. 2001;291:1284–1289. - PubMed
-
- Bohr VA, Sander M, Kraemer KH. DNA Repair (Amsterdam) 2004;4:293–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

