FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults
- PMID: 17229845
- PMCID: PMC1783105
- DOI: 10.1073/pnas.0607966104
FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults
Abstract
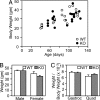
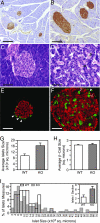
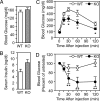
Activin and myostatin are related members of the TGF-beta growth factor superfamily. FSTL3 (Follistatin-like 3) is an activin and myostatin antagonist whose physiological role in adults remains to be determined. We found that homozygous FSTL3 knockout adults developed a distinct group of metabolic phenotypes, including increased pancreatic islet number and size, beta cell hyperplasia, decreased visceral fat mass, improved glucose tolerance, and enhanced insulin sensitivity, changes that might benefit obese, insulin-resistant patients. The mice also developed hepatic steatosis and mild hypertension but exhibited no alteration of muscle or body weight. This combination of phenotypes appears to arise from increased activin and myostatin bioactivity in specific tissues resulting from the absence of the FSTL3 antagonist. Thus, the enlarged islets and beta cell number likely result from increased activin action. Reduced visceral fat is consistent with a role for increased myostatin action in regulating fat deposition, which, in turn, may be partly responsible for the enhanced glucose tolerance and insulin sensitivity. Our results demonstrate that FSTL3 regulation of activin and myostatin is critical for normal adult metabolic homeostasis, suggesting that pharmacological manipulation of FSTL3 activity might simultaneously reduce visceral adiposity, increase beta cell mass, and improve insulin sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

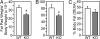
Similar articles
-
Follistatin and follistatin like-3 differentially regulate adiposity and glucose homeostasis.Obesity (Silver Spring). 2011 Oct;19(10):1940-9. doi: 10.1038/oby.2011.97. Epub 2011 May 5. Obesity (Silver Spring). 2011. PMID: 21546932 Free PMC article.
-
Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice.Metabolism. 2015 Feb;64(2):283-95. doi: 10.1016/j.metabol.2014.10.007. Epub 2014 Oct 14. Metabolism. 2015. PMID: 25456456
-
Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins.Endocrinology. 2006 Jul;147(7):3586-97. doi: 10.1210/en.2006-0089. Epub 2006 Apr 20. Endocrinology. 2006. PMID: 16627583
-
Differential actions of follistatin and follistatin-like 3.Mol Cell Endocrinol. 2004 Oct 15;225(1-2):25-8. doi: 10.1016/j.mce.2004.02.009. Mol Cell Endocrinol. 2004. PMID: 15451564 Review.
-
Roles of activin family in pancreatic development and homeostasis.Mol Cell Endocrinol. 2012 Aug 15;359(1-2):23-9. doi: 10.1016/j.mce.2012.02.015. Epub 2012 Mar 3. Mol Cell Endocrinol. 2012. PMID: 22406274 Review.
Cited by
-
FSTL3 is highly expressed in adipose tissue of individuals with overweight or obesity and is associated with inflammation.Obesity (Silver Spring). 2023 Jan;31(1):171-183. doi: 10.1002/oby.23598. Epub 2022 Dec 10. Obesity (Silver Spring). 2023. PMID: 36502285 Free PMC article.
-
Combined Inhibition of DYRK1A, SMAD, and Trithorax Pathways Synergizes to Induce Robust Replication in Adult Human Beta Cells.Cell Metab. 2019 Mar 5;29(3):638-652.e5. doi: 10.1016/j.cmet.2018.12.005. Epub 2018 Dec 20. Cell Metab. 2019. PMID: 30581122 Free PMC article.
-
The role of activin in mammary gland development and oncogenesis.J Mammary Gland Biol Neoplasia. 2011 Jun;16(2):117-26. doi: 10.1007/s10911-011-9214-4. Epub 2011 Apr 8. J Mammary Gland Biol Neoplasia. 2011. PMID: 21475961 Review.
-
A holistic approach to dissecting SPARC family protein complexity reveals FSTL-1 as an inhibitor of pancreatic cancer cell growth.Sci Rep. 2016 Nov 25;6:37839. doi: 10.1038/srep37839. Sci Rep. 2016. PMID: 27886258 Free PMC article.
-
Glycogen homeostasis and mtDNA expression require motor neuron to muscle TGFβ/Activin Signaling in Drosophila.bioRxiv [Preprint]. 2024 Jul 31:2024.06.25.600699. doi: 10.1101/2024.06.25.600699. bioRxiv. 2024. Update in: iScience. 2024 Dec 16;28(1):111611. doi: 10.1016/j.isci.2024.111611. PMID: 39131342 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

