DYRK1A autophosphorylation on serine residue 520 modulates its kinase activity via 14-3-3 binding
- PMID: 17229891
- PMCID: PMC1838983
- DOI: 10.1091/mbc.e06-08-0668
DYRK1A autophosphorylation on serine residue 520 modulates its kinase activity via 14-3-3 binding
Abstract
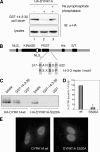
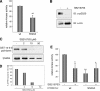
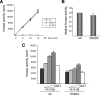
Dual-specificity tyrosine-phosphorylated and regulated kinase (DYRK) proteins are an evolutionarily conserved family of protein kinases, with members identified from yeast to humans, that participate in a variety of cellular processes. DYRKs are serine/threonine protein kinases that are activated by autophosphorylation on a tyrosine residue in the activation loop. The family member DYRK1A has been shown to phosphorylate several cytosolic proteins and a number of splicing and transcription factors, including members of the nuclear factor of activated T cells family. In the present study, we show that DYRK1A autophosphorylates, via an intramolecular mechanism, on Ser-520, in the PEST domain of the protein. We also show that phosphorylation of this residue, which we show is subjected to dynamic changes in vivo, mediates the interaction of DYRK1A with 14-3-3beta. A second 14-3-3 binding site is present within the N-terminal of the protein. In the context of the DYRK1A molecule, neither site can act independently of the other. Bacterially produced DYRK1A and the mutant DYRK1A/S520A have similar kinase activities, suggesting that Ser-520 phosphorylation does not affect the intrinsic kinase activity on its own. Instead, we demonstrate that this phosphorylation allows the binding of 14-3-3beta, which in turn stimulates the catalytic activity of DYRK1A. These findings provide evidence for a novel mechanism for the regulation of DYRK1A kinase activity.
Figures

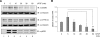


References
-
- Aitken A. 14-3-3 proteins: a historic overview. Semin. Cancer Biol. 2006;16:162–172. - PubMed
-
- Al-Hakim A. K., Goransson O., Deak M., Toth R., Campbell D. G., Morrice N. A., Prescott A. R., Alessi D. R. 14-3-3 cooperates with LKB1 to regulate the activity and localization of QSK and SIK. J. Cell Sci. 2005;118:5661–5673. - PubMed
-
- Altafaj X., et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Hum. Mol. Genet. 2001;10:1915–1923. - PubMed
-
- Alvarez M., Estivill X., de la Luna S. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J. Cell Sci. 2003;116:3099–3107. - PubMed
-
- Arron J. R., et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

