Gates and oscillators II: zeitgebers and the network model of the brain clock
- PMID: 17229921
- PMCID: PMC3281756
- DOI: 10.1177/0748730406296319
Gates and oscillators II: zeitgebers and the network model of the brain clock
Abstract
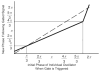
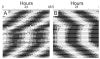
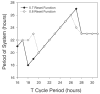
Circadian rhythms in physiology and behavior are regulated by the SCN. When assessed by expression of clock genes, at least 2 distinct functional cell types are discernible within the SCN: nonrhythmic, light-inducible, retinorecipient cells and rhythmic autonomous oscillator cells that are not directly retinorecipient. To predict the responses of the circadian system, the authors have proposed a model based on these biological properties. In this model, output of rhythmic oscillator cells regulates the activity of the gate cells. The gate cells provide a daily organizing signal that maintains phase coherence among the oscillator cells. In the absence of external stimuli, this arrangement yields a multicomponent system capable of producing a self-sustained consensus rhythm. This follow-up study considers how the system responds when the gate cells are activated by an external stimulus, simulating a response to an entraining (or phase-setting) signal. In this model, the authors find that the system can be entrained to periods within the circadian range, that the free-running system can be phase shifted by timed activation of the gate, and that the phase response curve for activation is similar to that observed when animals are exposed to a light pulse. Finally, exogenous triggering of the gate over a number of days can organize an arrhythmic system, simulating the light-dependent reappearance of rhythmicity in a population of disorganized, independent oscillators. The model demonstrates that a single mechanism (i.e., the output of gate cells) can account for not only free-running and entrained rhythmicity but also other circadian phenomena, including limits of entrainment, a PRC with both delay and advance zones, and the light-dependent reappearance of rhythmicity in an arrhythmic animal.
Figures

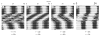
Comment in
-
Gate cells see the light.J Biol Rhythms. 2007 Feb;22(1):26-8. doi: 10.1177/0748730406297067. J Biol Rhythms. 2007. PMID: 17229922 No abstract available.
References
-
- Antle MC, Silver R. Orchestrating time: Arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

