Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells
- PMID: 17230217
- PMCID: PMC3232050
- DOI: 10.1038/ncpcardio0766
Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells
Abstract
Cardiogenesis within embryos or associated with heart repair requires stem cell differentiation into energetically competent, contracting cardiomyocytes. While it is widely accepted that the coordination of genetic circuits with developmental bioenergetics is critical to phenotype specification, the metabolic mechanisms that drive cardiac transformation are largely unknown. Here, we aim to define the energetic requirements for and the metabolic microenvironment needed to support the cardiac differentiation of embryonic stem cells. We demonstrate that anaerobic glycolytic metabolism, while sufficient for embryonic stem cell homeostasis, must be transformed into the more efficient mitochondrial oxidative metabolism to secure cardiac specification and excitation-contraction coupling. This energetic switch was programmed by rearrangement of the metabolic transcriptome that encodes components of glycolysis, fatty acid oxidation, the Krebs cycle, and the electron transport chain. Modifying the copy number of regulators of mitochondrial fusion and fission resulted in mitochondrial maturation and network expansion, which in turn provided an energetic continuum to supply nascent sarcomeres. Disrupting respiratory chain function prevented mitochondrial organization and compromised the energetic infrastructure, causing deficient sarcomerogenesis and contractile malfunction. Thus, establishment of the mitochondrial system and engagement of oxidative metabolism are prerequisites for the differentiation of stem cells into a functional cardiac phenotype. Mitochondria-dependent energetic circuits are thus critical regulators of de novo cardiogenesis and targets for heart regeneration.
Conflict of interest statement
The authors declared they have no competing interests.
Figures

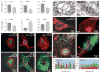
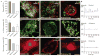
References
-
- Donovan PJ, Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97. - PubMed
-
- Behfar A, et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. - PubMed
-
- Perez-Terzic C, et al. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ Res. 2003;92:444–452. - PubMed
-
- Hodgson DM, et al. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol. 2004;287:H471–H479. - PubMed
-
- Van Laake LW, et al. Cardiomyocytes derived from stem cells. Ann Med. 2005;37:499–512. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
