Acid-base catalysis in Leuconostoc mesenteroides sucrose phosphorylase probed by site-directed mutagenesis and detailed kinetic comparison of wild-type and Glu237-->Gln mutant enzymes
- PMID: 17233628
- PMCID: PMC1876375
- DOI: 10.1042/BJ20070042
Acid-base catalysis in Leuconostoc mesenteroides sucrose phosphorylase probed by site-directed mutagenesis and detailed kinetic comparison of wild-type and Glu237-->Gln mutant enzymes
Abstract
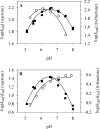
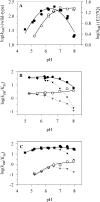
The role of acid-base catalysis in the two-step enzymatic mechanism of alpha-retaining glucosyl transfer by Leuconostoc mesenteroides sucrose phosphorylase has been examined through site-directed replacement of the putative catalytic Glu237 and detailed comparison of purified wild-type and Glu237-->Gln mutant enzymes using steady-state kinetics. Reactions with substrates requiring Brønsted catalytic assistance for glucosylation or deglucosylation were selectively slowed at the respective step, about 10(5)-fold, in E237Q. Azide, acetate and formate but not halides restored catalytic activity up to 300-fold in E237Q under conditions in which the deglucosylation step was rate-determining, and promoted production of the corresponding alpha-glucosides. In situ proton NMR studies of the chemical rescue of E237Q by acetate and formate revealed that enzymatically formed alpha-glucose 1-esters decomposed spontaneously via acyl group migration and hydrolysis. Using pH profiles of kcat/K(m), the pH dependences of kinetically isolated glucosylation and deglucosylation steps were analysed for wild-type and E237Q. Glucosylation of the wild-type proceeded optimally above and below apparent pK(a) values of about 5.6 and 7.2 respectively whereas deglucosylation was dependent on the apparent single ionization of a group of pK(a) approximately 5.8 that must be deprotonated for reaction. Glucosylation of E237Q was slowed below apparent pK(a) approximately 6.0 but had lost the high pH dependence of the wild-type. Deglucosylation of E237Q was pH-independent. The results allow unequivocal assignment of Glu237 as the catalytic acid-base of sucrose phosphorylase. They support a mechanism in which the pK(a) of Glu237 cycles between approximately 7.2 in free enzyme and approximately 5.8 in glucosyl enzyme intermediate, ensuring optimal participation of the glutamate residue side chain at each step in catalysis. Enzyme deglucosylation to an anionic nucleophile took place with Glu237 protonated or unprotonated. The results delineate how conserved active-site groups of retaining glycoside hydrolases can accommodate enzymatic function of a phosphorylase.
Figures



Similar articles
-
The role of Asp-295 in the catalytic mechanism of Leuconostoc mesenteroides sucrose phosphorylase probed with site-directed mutagenesis.FEBS Lett. 2007 Apr 3;581(7):1403-8. doi: 10.1016/j.febslet.2007.02.060. Epub 2007 Mar 5. FEBS Lett. 2007. PMID: 17350620
-
Asp-196-->Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glucosyl transfer to and from phosphate.FEBS Lett. 2006 Jul 10;580(16):3905-10. doi: 10.1016/j.febslet.2006.06.020. Epub 2006 Jun 19. FEBS Lett. 2006. PMID: 16797542
-
Dissecting differential binding of fructose and phosphate as leaving group/nucleophile of glucosyl transfer catalyzed by sucrose phosphorylase.FEBS Lett. 2007 Aug 7;581(20):3814-8. doi: 10.1016/j.febslet.2007.07.004. Epub 2007 Jul 16. FEBS Lett. 2007. PMID: 17659283
-
Mechanistic differences among retaining disaccharide phosphorylases: insights from kinetic analysis of active site mutants of sucrose phosphorylase and alpha,alpha-trehalose phosphorylase.Carbohydr Res. 2008 Aug 11;343(12):2032-40. doi: 10.1016/j.carres.2008.01.029. Epub 2008 Feb 2. Carbohydr Res. 2008. PMID: 18346723 Review.
-
[Application of sucrose phosphorylase in glycosylation].Sheng Wu Gong Cheng Xue Bao. 2021 Jan 25;37(1):112-129. doi: 10.13345/j.cjb.200213. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 33501794 Review. Chinese.
Cited by
-
Disaccharide phosphorylases: Structure, catalytic mechanisms and directed evolution.Synth Syst Biotechnol. 2021 Feb 13;6(1):23-31. doi: 10.1016/j.synbio.2021.01.004. eCollection 2021 Mar. Synth Syst Biotechnol. 2021. PMID: 33665389 Free PMC article.
-
Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase.Int J Mol Sci. 2019 Aug 11;20(16):3906. doi: 10.3390/ijms20163906. Int J Mol Sci. 2019. PMID: 31405215 Free PMC article.
-
Sucrose Phosphorylase and Related Enzymes in Glycoside Hydrolase Family 13: Discovery, Application and Engineering.Int J Mol Sci. 2020 Apr 5;21(7):2526. doi: 10.3390/ijms21072526. Int J Mol Sci. 2020. PMID: 32260541 Free PMC article. Review.
-
Energetics of the Glycosyl Transfer Reactions of Sucrose Phosphorylase.Biochemistry. 2023 Jun 20;62(12):1953-1963. doi: 10.1021/acs.biochem.3c00080. Epub 2023 May 30. Biochemistry. 2023. PMID: 37253063 Free PMC article.
-
The Structure of an Archaeal β-Glucosaminidase Provides Insight into Glycoside Hydrolase Evolution.J Biol Chem. 2017 Mar 24;292(12):4996-5006. doi: 10.1074/jbc.M116.766535. Epub 2017 Jan 27. J Biol Chem. 2017. PMID: 28130448 Free PMC article.
References
-
- Sinnott M. L. Catalytic mechanisms of enzymic glycosyl transfer. Chem. Rev. 1990;90:1171–1202.
-
- Davies G., Sinnott M. L., Withers S. G. Glycosyl transfer. In: Sinnott M. L., editor. Comprehensive Biological Catalysis. San Diego: Academic Press; 1998. pp. 119–208.
-
- Zechel D. L., Withers S. G. Glycosidase mechanisms: anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000;33:11–18. - PubMed
-
- Mieyal J. J., Abeles R. H. Disaccharide phosphorylases. In: Boyer P. D., editor. The Enzymes. New York: Academic Press; 1972. pp. 515–532.
-
- Schwarz A., Nidetzky B. Asp-196→Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glucosyl transfer to and from phosphate. FEBS Lett. 2006;580:3905–3910. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

