Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system
- PMID: 17233756
- PMCID: PMC2063589
- DOI: 10.1111/j.1600-0854.2006.00529.x
Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system
Abstract
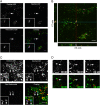
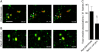
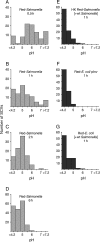
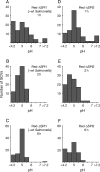
Following invasion of non-phagocytic host cells, Salmonella enterica survives and replicates within a phagosome-like compartment known as the Salmonella-containing vacuole (SCV). It is now well established that SCV biogenesis, like phagosome biogenesis, involves sequential interactions with the endocytic pathway. However, Salmonella is believed to limit these interactions and, in particular, to avoid fusion of terminal lysosomes with the SCV. In this study, we reassessed this process using a high-resolution live-cell imaging approach and found an unanticipated level of interaction between the SCV and the endocytic pathway. Direct interactions, in which late endosomal/lysosomal content was transferred to SCVs, were detected within 30 min of invasion and continued for several hours. Mechanistically, these interactions were very similar to phagosome-lysosome fusion because they were accompanied by rapid acidification of the SCV, could be blocked by chemical perturbation of microtubules or vacuolar acidification and involved the smallGTPase Rab7. In comparison with vacuoles containing internalized Escherichia coli or heat-killed Salmonella, SCVs did show some delay of fusion and acidification, although, this appeared to be independent of either type III secretion system. These results provide compelling evidence that inhibition of SCV-lysosome fusion is not the major determinant in establishment of the Salmonella replicative niche in epithelial cells.
Figures




Similar articles
-
The COPII complex and lysosomal VAMP7 determine intracellular Salmonella localization and growth.Cell Microbiol. 2015 Dec;17(12):1699-720. doi: 10.1111/cmi.12475. Epub 2015 Jul 16. Cell Microbiol. 2015. PMID: 26084942
-
The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells.EMBO J. 1999 Aug 16;18(16):4394-403. doi: 10.1093/emboj/18.16.4394. EMBO J. 1999. PMID: 10449405 Free PMC article.
-
Dynein-mediated vesicle transport controls intracellular Salmonella replication.Mol Biol Cell. 2004 Jun;15(6):2954-64. doi: 10.1091/mbc.e03-08-0614. Epub 2004 Apr 2. Mol Biol Cell. 2004. PMID: 15064357 Free PMC article.
-
How to do business with lysosomes: Salmonella leads the way.Curr Opin Microbiol. 2019 Feb;47:1-7. doi: 10.1016/j.mib.2018.10.003. Epub 2018 Nov 2. Curr Opin Microbiol. 2019. PMID: 30391777 Review.
-
Eat in or Take out? Metabolism of Intracellular Salmonella enterica.Trends Microbiol. 2020 Aug;28(8):644-654. doi: 10.1016/j.tim.2020.03.005. Epub 2020 Apr 25. Trends Microbiol. 2020. PMID: 32345466 Review.
Cited by
-
Role of host cell-derived amino acids in nutrition of intracellular Salmonella enterica.Infect Immun. 2015 Dec;83(12):4466-75. doi: 10.1128/IAI.00624-15. Epub 2015 Sep 8. Infect Immun. 2015. PMID: 26351287 Free PMC article.
-
Intestinal Epithelial Ecto-5'-Nucleotidase (CD73) Regulates Intestinal Colonization and Infection by Nontyphoidal Salmonella.Infect Immun. 2017 Sep 20;85(10):e01022-16. doi: 10.1128/IAI.01022-16. Print 2017 Oct. Infect Immun. 2017. PMID: 28717030 Free PMC article.
-
The Salmonella effector SseJ disrupts microtubule dynamics when ectopically expressed in normal rat kidney cells.PLoS One. 2017 Feb 24;12(2):e0172588. doi: 10.1371/journal.pone.0172588. eCollection 2017. PLoS One. 2017. PMID: 28235057 Free PMC article.
-
A novel anti-microbial function for a familiar Rab GTPase.Small GTPases. 2013 Oct-Dec;4(4):252-4. doi: 10.4161/sgtp.27282. Epub 2013 Dec 9. Small GTPases. 2013. PMID: 24321888 Free PMC article.
-
Epithelial cell extrusion: Pathways and pathologies.Semin Cell Dev Biol. 2017 Jul;67:132-140. doi: 10.1016/j.semcdb.2016.05.010. Epub 2016 May 19. Semin Cell Dev Biol. 2017. PMID: 27212253 Free PMC article. Review.
References
-
- Hensel M. Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol. 2004;294:95–102. - PubMed
-
- Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2000;2:145–156. - PubMed
-
- Knodler LA, Steele-Mortimer O. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic. 2003;4:587–599. - PubMed
-
- Patel JC, Galan JE. Manipulation of the host actin cytoskeleton by Salmonella– all in the name of entry. Curr Opin Microbiol. 2005;8:10–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

